Abstract
Background
Carotid intima media thickness (CIMT) and the presence of carotid plaque have been used for risk stratification of cardiovascular disease (CVD). To date, however, the association between multi-directional functional properties of carotid artery and CVD has not been fully elucidated. We sought to explore the multi-directional mechanics of the carotid artery in relation to cardiovascular risk.
Methods
Four hundred one patients who underwent carotid ultrasound were enrolled between January 2010 and April 2013. A high risk of CVD was defined as more than 20% of 10-year risk based on the Framingham risk score. Using a speckle-tracking technique, the longitudinal and radial movements were analyzed in the B-mode images. Peak longitudinal and radial displacements, strain and strain rate were also measured. Beta stiffness and elastic modulus index were calculated from the radial measurements.
Results
Of the overall sample, 13% (52) of patients comprised the high-risk group. In multivariate logistic regression, CIMT and elastic modulus index were independently associated with a high-risk of CVD {odds ratio (OR): 1.810 [95% confidence interval (CI) 1.249–2.622] and OR: 1.767 (95% CI: 1.177–2.652); p = 0.002, 0.006, respectively}. The combination of CIMT and elastic modulus index correlated with a high-risk of CVD more so than CIMT alone.
Conclusion
The elastic modulus index of the carotid artery might serve as a novel surrogate marker of high-risk CVD. Measurement of the multi-directional mechanics of the carotid artery using the speckle tracking technique has potential for providing further information over conventional B-mode ultrasound for stratification of CVD risk.
Atherosclerosis of the carotid artery is considered a useful marker of cardiovascular disease (CVD). Carotid intima media thickness (CIMT) as a surrogate marker of morphological pathology has frequently been employed for risk stratification of CVD by use of carotid ultrasound (CUS).1) Prior studies have documented both CIMT and parameters of arterial stiffness are associated with hypertension (HTN) coronary atherosclerosis, stroke, and cardiovascular mortality.2)3)4)5) Further still, CIMT is routinely recommended for evaluation of end organ damage in patients with HTN based on current guidelines.6) CIMT reflects the morphological changes of the arterial wall which associated with atherosclerosis, while the parameters of arterial stiffness tend to reflect the functional properties of the carotid arteries and display changes prior to the development of structural alterations of the arterial wall including increased CIMT or carotid plaque.7) Arterial stiffness can be measured non-invasively by B-mode ultrasound while utilizing specialized software.8) To date, however, the prognostic utility of functional analysis of the carotid artery to the conventional CIMT measurement remains to be fully understood. Notably, the strain imaging with speckle tracking method measures multi-directional movement throughout the cardiac cycle, and is capable of directly analyzing the elasticity of the carotid artery. Therefore, in this study, we sought to examine the additional value of multi-directional functional mechanics of the carotid artery in relation to cardiovascular risk.
Among a consecutive series of 419 patients who visited a single cardiology outpatient clinic, 18 patients were excluded due to a history of carotid stenting (n = 3), and diffuse multiple carotid plaque (n = 15). In total, 401 consecutive patients who were enrolled from January 2010 to April 2013 underwent CUS. All patients were analyzed for multi-directional mechanics of the carotid artery, using the speckle-tracking technique.
The Framingham risk score (FRS) was calculated for all patients. 9) Cardiovascular risk factors such as a history of HTN, diabetes mellitus (DM), and smoking were obtained by review of medical records.
HTN was defined as blood pressure (BP) ≥ 140/90 mm Hg by brachial BP or the use of antihypertensive medications. DM was defined as a fasting glucose level ≥ 126 mg/dL or random glucose level > 200 mg/dL, with symptoms of hyperglycemia such as polydipsia or polyuria, or the use of an oral hypoglycemic agent or insulin. Smoking was calculated as the number of pack-years and categorized into three groups including: non-smoker, ex-smoker and current smoker. A high risk of CVD was defined as more than 20% of 10-year risk based on FRS.
High-resolution B-mode CUS was performed using linear array transducer (nominal band width of 3–12 MHz) with 8 MHz center frequency [iE33 echo machine (Philips Medical Systems, Andover, MA, USA) or Vivid7 scanner (GE Medical-Systems, Horten, Norway)]. To measure accurately the movement of carotid artery, the view angle was reduced to acquire a high frame rate, which was above 120 fps. Both common carotid arteries (CCA) were examined with the head tilted slightly upward in the midline position. At least two consecutive beats were stored and the multi-directional movement of the carotid artery data was analyzed by arterial analysis software (Samsung Medison Co., Ltd., Seoul, Korea).
The arterial analysis software evaluates the functional ability of the vessels by calculating automatically the displacements of the wall motion. The user defined control points, which represent the wall of the vessel at an arbitrary frame, are tracked automatically based on an optical flow algorithm.10) To track the motion with stability, the control points are constrained to maintain the global shape and to move within a local range. The tracking performance was evaluated by two experiments. The first experiment was conducted to assess the accuracy of the tracking algorithm by using a synthetic data. The phantom of the dynamic vessel, at both longitudinal and transversal view, was made by using an ultrasound simulator. The second experiment was conducted to evaluate the tracking ability on various clinical data by comparing the manually and automatically measured diameter differences (ΔD).
The mean value of CIMT was measured semi-automatically at the CCA in the longitudinal plane where the plaques were not included at end-diastole phase, at least 5 mm proximal to the carotid bulb, and approximately 10 mm in CCA length. The mean value of CIMT at CCA was measured and the average value of CIMT in the right and left CCA was used in analysis. The carotid plaque was defined as a focal structure protruding into the arterial lumen of at least 1.5 mm or 50% of the surrounding IMT value. For analysis, images of the patients presenting with carotid plaque were acquired at another close location at the common carotid artery where the plaques were not included. A minimum of four speckles on the carotid artery wall were identified and the movements of each speckle during the cardiac cycle were traced at the near and far walls.
For the study analysis, at least 5 mm of the CCA below the origin of the carotid bulb was used. Both near and far wall interfaces defining the blood-intima boundaries or carotid artery were manually traced from a still frame image and automatically tracked by the software. Based on the speckle-tracking technique, longitudinal and radial movements of the carotid artery were calculated automatically in the longitudinal plane. In addition, peak longitudinal and radial displacements, strain, and strain rate were measured. Parameters of arterial stiffness such as arterial compliance, distensibility, elastic modulus, and beta stiffness index were calculated using arterial analysis software from the radial measurements. Central BP was used for calculation of the formula. Central BP was measured with pulse wave analysis of the radial artery using tonometry (SphygmoCor, AtCorMedical, Sydney, Australia) by trained technician. The measurements were obtained at the wrist after subjects had rested for 5 min in the supine position. From the radial signal, the SphygmoCor software calculates the aortic pulse wave by means of a generalized transfer function. The central BPs were derived from the aortic pulse wave.
Arterial compliance is the absolute change in vessel diameter for a given change in pressure [e.g., difference between diastolic diameter and systolic diameter (ΔD) / difference between systolic blood pressure (SBP) and diastolic blood pressure (DBP) (ΔP)]. Distensibility is the relative change in vessel diameter for a given change in pressure {e.g., ΔD / [ΔP × vessel diameter in diastolic phase (D)] mm Hg–}. Elastic modulus index is the pressure change required for theoretical stretch from resting diameter [e.g., (ΔP × D) / ΔD mm Hg] (Fig. 1). Beta stiffness index is the ratio of the natural logarithm of systolic/diastolic blood pressure to relative change in diameter [log (SBP/DBP) / (ΔD/D)].11)12)13)14) All parameters of arterial stiffness were automatically calculated using arterial analysis software (Fig. 2).
Continuous variables are expressed as mean ± standard deviation. Differences in clinical data, parameters for multi-directional mechanics of the carotid artery, CIMT and the presence of carotid plaque between patients with and without high risk of CVD were assessed using the χ2 test and independent t-tests. A binary logistic regression analysis was performed to assess the association of the high-risk group according to the FRS with sex, age, HTN, DM, carotid plaque, CIMT, and variable parameters for assessment of multi-directional movement of the carotid artery. In additional analysis, we used receiver operating characteristic (ROC) curves to determine the cut-off values of predictors. All statistical analysis was performed using the statistical software program SPSS for Windows (version 18.0; SPSS Inc., Chicago, IL, USA). A two-sided p-value of ≤ 0.05 was considered significant.
The mean age of patients was 62 ± 11 years and 69% (n = 276) were men. Among 401 study patients, 13% (n = 52) were at high risk of CVD according to FRS (Table 1). The study population was divided into a high-risk group and a non-high risk group based on FRS. The patients within the high-risk group tended to be older than patients belonging to the non-high risk group (mean age: 70.3 ± 7.6 years vs. 60.5 ± 10.9 years, p < 0.001). Further, the high-risk group had a larger male-to-female ratio than the non-high risk group [e.g., 96% (n = 50) vs. 65% (n = 226), p < 0.001]. Likewise, there were more smokers in the high-risk group than in the non high-risk group [96% (n = 50) vs. 52% (n = 183), p < 0.001] (Table 2). Conversely, there were no significant differences in HTN or DM between groups.
Carotid plaques were identified in 79% of the high-risk group patients and there were more carotid plaques in the high-risk group than in the non high-risk group. Mean CIMT was 0.93 ± 0.19 mm in the high-risk group and 0.77 ± 0.18 mm in the non high-risk group (p < 0.001). Among the parameters of arterial stiffness, elastic modulus index, beta stiffness index and longitudinal displacement were found to be significantly larger in the high-risk group than in the non high-risk group. On the other hand, longitudinal and radial strain showed no significant differences between groups (Table 3).
In multivariate logistic regression, CIMT and elastic modulus index were independently associated with a high risk of CVD {odds ratio (OR): 1.810 [95% confidence interval (CI) 1.249–2.622] and OR: 1.767 (95% CI: 1.177–2.652); p = 0.002, 0.006, respectively} (Table 4). The intra- and inter-class correlation coefficient of elastic modulus index between the 10 selected cases was 0.95 and 0.93, respectively.
Furthermore, given the remarkable differences encountered in men and women, we analyzed multiple logistic regression separately in men and women. In men, CIMT and elastic modulus index were independently associated with a high risk of CVD (OR: 1.383 and OR: 1.795; p < 0.001, 0.001, respectively). However, there are only two people of high risk in women, and there were no parameter of arterial stiffness which have significant association with high risk of CVD in women.
Since ultrasound biomarkers of CV risk are useful in low to intermediate CV risk, we analyzed FRS as continuous variable while excluding those in the high risk group. When analyzing the linear regression model excluding high-risk patients, CIMT and elastic modulus remained associated with continuous FRS.
In additional analyses, we evaluated the association of ultrasonographic parameters of the carotid artery with high risk of CVD by use of a ROC curve. In Fig. 3, the area under the ROC curve for elastic modulus index (0.712, 95% CI: 0.639–0.785) is similar with that of CIMT (0.737, 95% CI 0.668–0.805).
Given CIMT is a well-known parameter with respect to CVD, we subsequently compared the area under the curve (AUC) of CIMT with the AUC of CIMT in combination with elastic modulus index to determine the high-risk group of CVD. We found the latter combination of CIMT and elastic modulus index had a significantly greater AUC for the high-risk group of CVD than CIMT alone (e.g., AUC: 0.794 vs. 0.737, p = 0.002) (Fig. 4). Further still, the combination of CIMT and elastic modulus index correlated with CVD more so than CIMT alone.
The current study set out to evaluate the added value of multi-directional functional mechanics of the carotid artery with regards to cardiovascular risk. The main findings were that 1) multi-directional mechanics of carotid artery were capable of being analyzed by speckle tracking; 2) the elastic modulus index of the carotid artery and CIMT were independently associated with the high-risk CVD group; and 3) the elastic modulus index of the carotid artery have additional value in terms of relationship with high-risk CVD.
This study evaluated the ultrasonographic speckle-tracking-based strain values, the parameters of arterial stiffness, and their relationship with the high risk of CVD in patients who underwent CUS. The multi-directional mechanics of the carotid artery including strain values and the parameters of arterial stiffness were assessed using speckle-tracking software. Numerous previous studies have attempted to report the relationship between atherosclerotic CVD and arterial strain by using the speckle-tracking method. Indeed, one study documented that the change in elastic properties as a consequence of aging could be successfully evaluated with carotid arterial strain values acquired by the speckle tracking method.15) More recently, several other studies have reported that ultrasound-based carotid arterial strain can be measured using speckle tracking,16)17) and that carotid arterial strain measured using speckle tracking was lower in patients with DM. To this end, speckle tracking may be a more sensitive measure than more conventional methods of the carotid artery.18) Recent studies have also assessed the longitudinal movement of the CCA wall with B-mode ultrasound imaging in vivo using a speckle tracking method.19) In this study, the longitudinal and radial movement of the carotid artery including strain values and the various parameters of arterial stiffness were assessed using a longitudinal view of the carotid artery. Arterial stiffness measures can be assessed from CUS with a relatively simple additional analysis of arterial movement by speckle tracking method. Advances in imaging technology of CUS may allow for more accurate estimation of the arterial dimensions in multiple planes, thus further improving assessment of arterial stiffness. Therefore, the measurement of multi-directional functional properties of the carotid artery using a speckle tracking technique may serve as a useful tool for providing incremental information beyond the conventional B-mode ultrasound for risk stratification of CVD.
In this study, the elastic modulus index, beta stiffness index, and longitudinal displacement were significantly larger in the high-risk group as compared with the non high-risk group. Many previous studies reported that these parameters of arterial stiffness were associated with a heightened risk of CVD. Blacher et al.20) reported that the increased elastic modulus is a strong predictor of cardiovascular mortality in patients with end stage renal disease. Riley et al.21) demonstrated that Young's elastic modulus provided more information into the development of CVD in a large population-based study. Carotid distensibility has been regarded as a novel parameter for CVD in population-based cohorts, including the Atherosclerosis Risk in Communities Study,22) Second Manifestations of Arterial Disease, the Rotterdam Study,23) the Baltimore Longitudinal Study of Aging, and the Multiethnic Study of Atherosclerosis.24) Another recent study showed that the severity of coronary artery disease was significantly correlated with strain and strain rate.7) With aging, the functional and structural changes in the carotid artery characteristic of stiffness lead to increases in SBP, which usually results in a slight decrease in DBP, and a marked increase in pulse pressure. For these reasons, it is possible that the elastic modulus index and beta stiffness index were found to increase with aging and high risk of FRS in this study.
Functional impairments of the arterial wall may appear in the early stages of atherosclerosis before the occurrence of detectible structural changes, as well as prior to the development of clinical symptoms of CVD.25) Conventional methods for the measurement of arterial stiffness such as the elastic modulus and distensibility26) showed early changes before the development of clinical symptoms or visible atherosclerotic plaques.26)27) Foremost, early detection of functional impairments may provide more effective approaches for the prevention of CVD. In addition, traditional risk factors often fail to predict major cardiovascular events.28) Thus, new indices of early arterial wall alteration appear necessary. In this study, elastic modulus index was independently associated with high risk of CVD after adjusting for CIMT and the presence of carotid plaque.
The elastic modulus index provided additional predictive value above CIMT for the association with high risk of CVD. The elastic modulus has previously been reported as a strong predictor of cardiovascular mortality,20) which could provide further information towards the development of CVD.21) This study also demonstrated that the value of the AUC significantly improved after combining CIMT with elastic modulus index (AUC 0.556 vs. 0.683, p = 0.002) (Fig. 3), indicating that the combination of these parameters may prove useful for improving the ability to define a high risk of CVD prior to the occurrence of a cardiovascular event.
The present study has some limitations that should be emphasized. The retrospective design and single-center nature of the study limited our analysis. Assessments of multi-directional mechanics along the longitudinal plane only were recorded. In addition, stiffness measurements were estimated using data from the CCA and only reflect the characteristics of that region of the carotid artery. In total, only 13% of the study participants comprised the high-risk CVD group, which is perhaps relatively small compared to the general population. Thus, the current study findings may not be fully representative of the characteristics of strain values and arterial stiffness in the general population. Further, the high-risk group consisted of relatively more men than the non-high risk group. Men tend to have a larger diameter of carotid artery than women, which may have also affected the value of elastic modulus. Last, there were no parameter of arterial stiffness which have significant association with high risk of CVD in women group because only two people of high risk were in women group.
The findings of this study indicate that a larger elastic modulus index is associated with a high risk of CVD. The combination of CIMT and elastic modulus index significantly improved the ability to define a high risk of CVD in its early stage, before the occurrence of a cardiovascular event. The supplementary assessment of the mechanical property with CIMT by specialized software lended additional value than that of the CIMT measurement alone. The assessment of mechanical property of the carotid artery can be performed relatively quickly and calculated automatically by software. Based on the current study findings, measuring the mechanical property of the carotid artery wall by speckle tracking method has potential to provide useful information towards risk stratification of CVD.
Figures and Tables
Fig. 2
Examples of carotid echocardiographic data from B-mode (left) and longitudinal strain curves of the carotid artery by speckle-tracking imaging (right) in a 45-year-old woman belonging to the non high-risk group (A) and a 48-year-old woman in the high-risk group (B). IMT: intima media thickness.

Fig. 3
Receiver operating characteristic (ROC) curves for CIMT (A) and elastic modulus index (B). IMT: intima media thickness, CIMT: carotid intima media thickness.

Fig. 4
Receiver operating characteristic (ROC) curves for CIMT, elastic modulus index and CIMT + elastic modulus index. *IMT = 0.7367, †Elastic modulus = 0.7122, ‡IMT + elastic modulus = 0.7943. IMT: intima media thickness, CIMT: carotid intima media thickness.

Table 2
Comparison of baseline characteristics according to the risk of CVD
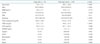
Data are expressed as number (%) or mean ± standard deviation. BMI: body mass index, HTN: hypertension, DM: diabetes mellitus, HDL: high density lipoprotein, LDL: low density lipoprotein, hsCRP: high sensitive C-reactive protein, ACEi: angiotensin converting enzyme inhibitor, CCB: calcium channel blocker, CVD: cardiovascular disease
Acknowledgements
This research was supported by the Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (MSIP) (No. 2012027176).
References
1. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999; 340:14–22.
2. Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004; 109:184–189.
3. Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005; 111:3384–3390.
4. Mancini GB, Dahlöf B, Díez J. Surrogate markers for cardiovascular disease: structural markers. Circulation. 2004; 109:25 Suppl 1. IV22–IV30.
5. Myung Y, Seo HS, Jung IH, Lee NH, Suh J, Choi JH, Cho YH. The correlation of carotid artery stiffness with heart function in hypertensive patients. J Cardiovasc Ultrasound. 2012; 20:134–139.
6. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013; 31:1281–1357.
7. Kim SA, Park SM, Kim MN, Kim YH, Cho DH, Ahn CM, Hong SJ, Lim DS, Shim WJ. The relationship between mechanical properties of carotid artery and coronary artery disease. Eur Heart J Cardiovasc Imaging. 2012; 13:568–573.
8. Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003; 23:554–566.
9. D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P. CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001; 286:180–187.
10. Veronesi F, Corsi C, Caiani EG, Sarti A, Lamberti C. Tracking of left ventricular long axis from real-time three-dimensional echocardiography using optical flow techniques. IEEE Trans Inf Technol Biomed. 2006; 10:174–181.
11. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006; 27:2588–2605.
12. Sugawara M, Niki K, Furuhata H, Ohnishi S, Suzuki S. Relationship between the pressure and diameter of the carotid artery in humans. Heart Vessels. 2000; 15:49–51.
13. Laurent S, Caviezel B, Beck L, Girerd X, Billaud E, Boutouyrie P, Hoeks A, Safar M. Carotid artery distensibility and distending pressure in hypertensive humans. Hypertension. 1994; 23(6 Pt 2):878–883.
14. Gamble G, Zorn J, Sanders G, MacMahon S, Sharpe N. Estimation of arterial stiffness, compliance, and distensibility from M-mode ultrasound measurements of the common carotid artery. Stroke. 1994; 25:11–16.
15. Bjällmark A, Lind B, Peolsson M, Shahgaldi K, Brodin LA, Nowak J. Ultrasonographic strain imaging is superior to conventional non-invasive measures of vascular stiffness in the detection of age-dependent differences in the mechanical properties of the common carotid artery. Eur J Echocardiogr. 2010; 11:630–636.
16. Zahnd G, Vray D, Sérusclat A, Alibay D, Bartold M, Brown A, Durand M, Jamieson LM, Kapellas K, Maple-Brown LJ, O'Dea K, Moulin P, Celermajer DS, Skilton MR. Longitudinal displacement of the carotid wall and cardiovascular risk factors: associations with aging, adiposity, blood pressure and periodontal disease independent of cross-sectional distensibility and intima-media thickness. Ultrasound Med Biol. 2012; 38:1705–1715.
17. Zahnd G, Boussel L, Marion A, Durand M, Moulin P, Sérusclat A, Vray D. Measurement of two-dimensional movement parameters of the carotid artery wall for early detection of arteriosclerosis: a preliminary clinical study. Ultrasound Med Biol. 2011; 37:1421–1429.
18. Yang EY, Dokainish H, Virani SS, Misra A, Pritchett AM, Lakkis N, Brunner G, Bobek J, McCulloch ML, Hartley CJ, Ballantyne CM, Nagueh SF, Nambi V. Segmental analysis of carotid arterial strain using speckle-tracking. J Am Soc Echocardiogr. 2011; 24:1276–1284.e5.
19. Golemati S, Sassano A, Lever MJ, Bharath AA, Dhanjil S, Nicolaides AN. Carotid artery wall motion estimated from B-mode ultrasound using region tracking and block matching. Ultrasound Med Biol. 2003; 29:387–399.
20. Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension. 1998; 32:570–574.
21. Riley WA, Barnes RW, Evans GW, Burke GL. Ultrasonic measurement of the elastic modulus of the common carotid artery. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 1992; 23:952–956.
22. Riley WA, Evans GW, Sharrett AR, Burke GL, Barnes RW. Variation of common carotid artery elasticity with intimal-medial thickness: the ARIC Study. Atherosclerosis Risk in Communities. Ultrasound Med Biol. 1997; 23:157–164.
23. van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001; 32:454–460.
24. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002; 156:871–881.
25. Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001; 154:185–193.
26. Kawasaki T, Sasayama S, Yagi S, Asakawa T, Hirai T. Non-invasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc Res. 1987; 21:678–687.
27. Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006; 113:657–663.
28. Helfand M, Buckley DI, Freeman M, Fu R, Rogers K, Fleming C, Humphrey LL. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009; 151:496–507.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download