Abstract
The appropriate use of echocardiography may reduce the need for invasive diagnostic cardiac procedures. The right side of the heart has recently gained interest among cardiologists as it became clear that abnormalities of the right heart morphology and function are associated with increased morbidity and mortality. Echocardiography is easy to perform, relatively cheap, readily available and do not pose the risk of ionizing radiation. Conventional 2D and, more recently, 3D echocardiography provides pertinent anatomic and physiologic information about the right side of the heart. Because of the advantages and simplicity of echocardiography it continues to be an excellent tool for evaluating the structure and function of the right side of the heart. This review outlines the uses of echocardiography in evaluating the right heart structure and function.
The right ventricle (RV) is anterior to the left ventricle (LV) and behind the sternum which has implications for its specific morbidity and mortality but which also makes it potentially accessible to surface imaging such as transthoracic echocardiography. Assessment of right heart function remains relatively difficult despite technological advancement in echocardiography because of the unusual crescent shape, the irregular endocardial surface, complex contraction mechanism and location of the RV.1)2) However, the introduction of Doppler and color Doppler techniques as well as improvements in image quality and imaging modalities has made it possible to use echocardiography as a modality to assess the structure and function of the right heart.3)4)5) Jaussi et al.6) concluded that it is prudent to evaluate the right heart structures in detail as the prevalence of right heart abnormalities found on routine echocardiography is significant.
The RV is immediately behind the sternum and is the most anterior part of the heart which is bordered by the annulus of the tricuspid and pulmonary valve. It is crescent shaped in cross sectional view and triangle shaped in the side view (Fig. 1).7) Anatomically, the RV consists of inflow tract, the apex and infundibulum.5)8) There are three prominent bands present in the RV of which the moderator band distinguishes RV from LV.5)9) The ventriculoinfundibular fold is another important structure that separates the tricuspid and pulmonary valves, which is in contrast to the LV where the aortic and mitral valves are in fibrous continuity. When assessing the RV volume the complex shape and infundibulum should be included.10) The shape can also be influenced by the septum which is concave toward the LV in both systole and diastole. The normal RV measurements by 2D echocardiography are; base apex length less than 8.6 cm, midcavity diameter less than 3.5 cm, basal diameter less than 4.2 cm, RV outlet parasternal short axis 2.7 cm, RV outlet parasternal long axis 3.3 cm, and end diastolic area less than 28 cm2.1)4).
There are different modalities to assess RV function (Table 1). The RV ejection fraction is driven by right ventricular preload, after load and contractility.11) The tricuspid annular plane systolic excursion is correlated to RV ejection fraction12) which has a mean value of 52.3 ± 6.2% with 40% being the lower limit of normal and there is about 5% increase during exercise13) and is used as a surrogate for global RV systolic function. RV dimensions and function can be measured by standard M-mode, 2D echocardiography and Doppler echocardiography, the findings correlate with ejection fraction derived by radionuclide angiography.14) Another method for measuring the RV function is myocardial performance index or the Tei index. It is calculated as the ratio between total RV isovolumic time (contraction and relaxation) divided by pulmonary ejection time. The RV myocardial performance index has been shown to correlate with pulmonary pressures and can be used to identify RV dysfunction.15)16)17) The normal RV systolic functional values by echocardiography; tricuspid annular plane excursion greater than 16 mm, fractional area change greater than 35%, and peak systolic tissue Doppler velocity at tricuspid annulus greater than 10 cm per second. The diastolic RV function values by echocardiography; tricuspid E/A ratio less than 0.8 is consistent with decreased relaxation, tricuspid E/A ratio 0.8 to 2.1 with E/E' ratio greater than 6 is consistent with falsely normal filling, and tricuspid E/A ratio greater than 2.1 with deceleration lime less than 120 msec is consistent with restrictive filling.1)4)
Doppler tissue imaging is set to detect low velocity with high amplitude, which provides accurate information on myocardial motion throughout the cardiac cycle, detects myocardial tissue motion as opposed to red cells and is used to derive strain and strain rate, both of which are less preload dependent as compared to traditional ejection fraction.18)19)20)21) 3D echocardiography may have a potential advantage in determining RV volumes and image reconstruction; however this technique remains limited because of poor lateral resolution in right ventricular cavity dilatation.22) Due to limited studies it is recommended that 3D echocardiography can be utilized only for serial volume and ejection fraction evaluation with the RV ejection fraction lower limit set at 44% as this modality has lesser underestimation than 2D echocardiography when compared to cardiac magnetic resonance imaging.23)24) In recent years real time 3D echocardiography has been found to be a valuable tool for assessing the RV anatomy and function. 3D echocardiography is reproducible, easily available, and accurate despite the geometric challenges placed by the RV structure and position.25)26)
The right atrium not only acts as a conduit to fill the RV passively but also optimizes RV filling actively during late diastole. Anatomical deformities of the spine and chest can alter the right atrium and project larger dimensions. Echocardiographic four chamber apical views are used to evaluate the right atrium and the subcostal views to evaluate the inferior vena cava.
RV systolic function is a reflection of contractility, afterload, and preload. RV performance is also influenced by heart rhythm, synchrony of ventricular contraction, RV force interval relationship, and ventricular interdependence.27)
RV pressure overload can be due to obstruction of the RV outflow tract (pulmonary stenosis), primary pulmonary hypertension or secondary pulmonary hypertension. In the absence of RV outflow tract obstruction, the tricuspid regurgitation jet can be used to assess the pulmonary artery systolic pressure.28) Endsystolic flattening of the interventricular septum is an indication of RV pressure overload. With chronic increase in afterload the RV dilates and hypertrophies, the tricuspid annulus might also dilate and cause severe tricuspid regurgitation. Right atrial area index, diastolic eccentricity index and pericardial effusion score are indicators of morbidity and potential mortality.29) RV hypertrophy is defined as the RV free wall thickness greater than 5–7 mm measured at the end-diastole by M-mode or 2D echocardiography from either parasternal long axis or subcostal window.
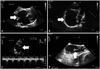
RV volume overload can be result of left to right shunting as in atrial septal defect (Fig. 2A), pulmonary anomalous venous return or ventricular septal defect.30) Significant valvular regurgitation can also contribute to RV volume overload. Color Doppler studies, venous injection of microbubbles as well as image acquisition in the subcostal view or transesophageal echocardiography are often useful tools in echocardiography to assess RV structure and function in a volume overload state. When the right ventricular volume is high the LV acquires a D shape in diastole as result of the interventricular interdependence which can be visualized by echocardiography.1)3)
RV diastolic dysfunction is an independent predictor of mortality31) with a complex pathophysiology which can be evaluated by the trans-tricuspid E/A ratio, the E/E' ratio, and the right atrial size.1)
Right atrial echocardiographic measurements take longer time that most other measurements, but are valuable in assessing RV and LV dysfunction.1)4) Lower global longitudinal strain of RV (≥ -15.5%) in patients with pulmonary arterial hypertension measured with velocity vector imaging using 2D strain echocardiographic measurement was found to have increased mortality.32) 3D and 2D echocardiographic apical four chamber planimetry area is utilized for evaluating right atrial volume. 33) In patients with primary pulmonary hypertension right atrial area is a predictor of transplantation or mortality. Patients on mechanical ventilators pose challenges in evaluating the right atrium in the setting of inferior vena caval collapse. The normal right atrial measurements by 2D echocardiography are; minor dimension less than 4.4 cm, major dimension less than 5.3 cm, and end systolic area less than 20 cm2.1)4)
The structural changes in the right heart can be due to congenital anomalies, valvular disease, myocardial disease or postoperative changes (Fig. 2A).
The echocardiography is useful in diagnosing and following congenital heart disease because it is easy to perform, readily available, relatively inexpensive, although magnetic resonance imaging is used more and more for these patients.34) Appropriate use of echocardiography may also decrease the need for invasive diagnostic procedures.35) In congenital heart disease, the RV may support the pulmonary or systemic circulation and can lead to RV dysfunction over time which is known to be associated with increased morbidity and mortality and therefore will need close monitoring and surveillance. The combination of 2D echocardiography and Doppler can provide extensive information on the anatomy and function of the heart.30)36)
The important congenital anomalies affecting the right ventricular inflow tract and tricuspid valve can be diagnosed by specific findings on echocardiography. Tricuspid atresia is a condition in which the lack of development of the RV leads to low pulmonary flow in addition to the imperforate valvular membrane between the floor of the right atrium and the hypoplastic RV. 2D echocardiography shows an echo-dense band of fibrofatty tissue of the atrioventricular groove and the absent right atrioventricular connection.37) In patients with Ebstein's anomaly (Fig. 2B), the echocardiography will reveal an apical displacement of the septal leaflet of the tricuspid valve, tethering of the tricuspid valve causing restricted motion of the leaflet, marked enlargement of the right atrium (with an atrialized RV), and tricuspid regurgitation.27) The underdevelopment of the right ventricular myocardium, or as Uhl's disease, leads to right ventricular dilatation and eventually right ventricular failure.
The right ventricular outflow tract and the corresponding anomalies can be visualized by echocardiography in a similar fashion. Subvalvular stenosis usually involving the infundibulum results from fibromuscular narrowing or hypertrophied subvalvular muscle bundles also called double-chambered RV.38) Double-chambered RV is difficult to visualize with echocardiography and subcostal echocardiography may provide optimal visualization.39)40) Typically subvalvular stenosis is a dynamic form of obstruction with maximal flow velocity occurring in late systole. Valvular pulmonary stenosis may lead to ventricular hypertrophy and subsequent outflow tract narrowing. In this form of obstruction, using 2D echocardiography, the pulmonary cusps appear thickened, have a decreased excursion and dome in systole with a resulting increased peak instantaneous pressure gradient (Fig. 2C); however, this technique is limited for assessing the severity of obstruction.41)42) Furthermore, the pulmonary arteries should always be assessed in children with right ventricular outflow tract obstruction because the development of the pulmonary arteries can be affected by the flow and pulmonary artery stenosis can be associated with other congenital cardiac and extracardiac lesions.43) Color Doppler imaging can be used to assess the turbulence and acceleration of flow within the stenotic segment.
Arrhythmogenic right ventricular dysplasia, a congenital autosomal dominant myocardial disease that is characterized by progressive fibrofatty replacement of the myocardium, can lead to ventricular tachycardia and sudden death in young people and especially in athletes. Diagnosis of arrhythmogenic right ventricular dysplasia can be made by variety of invasive and non-invasive modalities but magnetic resonance imaging is considered the gold standard. 2D echocardiographic findings suggestive of arrhythmogenic right ventricular dysplasia are RV dilatation, altered myocardial texture, localized RV aneurysms and inferobasal or localized dyskinesia.44)45) 3D echocardiography is a useful modality; however, currently interpretations are variable based on the centers and guidelines have not been established.
Tetralogy of Fallot is the most common cyanotic congenital heart disease. This anomaly has four anatomical features: 1) anterior and rightward displacement of the aortic root, 2) ventricular septal defect, 3) right ventricular outflow obstruction, and 4) right ventricular hypertrophy.46)47) Echocardiography can be used for de novo diagnosis of the lesion, in determining options for surgical intervention and postoperative assessment of the adequacy of repair. In tetralogy of Fallot, the most critical defect is the mal-alignment of the infundibular septum, resulting in septal defect and overriding aorta. This is optimally visualized in the parasternal long-axis view. The short axisview allows for determining the extent and size of the septal defect in addition to assessment of the right ventricular outflow tract.46)47)48) Color Doppler is useful in assessing the location of the narrowed, turbulent flow, and continuous wave Doppler can then be used to determine the pressure gradient across the obstruction. Additionally coronary artery anatomy must be examined before surgery. In addition to coronary angiography, this can also be done by using 2D echocardiography techniques such as visualizing a coronary artery branch across the right ventricular outflow tract.46)47)48) Postoperatively the ventricular septal defect patch can be seen in the parasternal long-axis view as the linear structure passing obliquely from septum to anterior aortic root, shunting can be evaluated using Doppler, and right ventricular size and contractility should be assessed. The RV volume is better evaluated by 3D echocardiography in post tetralogy of Fallot surgery.49)
Transposition of the great arteries is another common congenital heart disease which refers to the aorta arising from the RV and the pulmonary artery arising from the LV. The parasternal long-axis view demonstrates normal ventricular relationship with ventriculoarterial discordance. The diagnosis of D-transposition is confirmed in the subcostal four chamber view where a bifurcating great artery is seen arising from the LV as the pulmonary artery.36)50)51)52) The current standard for correction of this anatomic anomaly is an arterial switch where both great arteries are transected and re-anastomosed to the correct ventricle. Tissue Doppler echocardiography with strain and strain rate imaging is a good modality to evaluate myocardial function in patients who have undergone correction of transposition of great arteries.36) Postoperative echocardiographic evaluation should focus on assessment of the left and right ventricular function, valvular narrowings, anastomotic site, and the origin of the coronaries.53)
Double-outlet RV refers to congenital heart disease with an anomalous bundle of muscle which divides the right heart into a high pressure inlet and a low pressure outlet, both great arteries arise from the RV and ventricular septal defect acts as the only outlet for the LV. Echocardiographic diagnosis is based on demonstration of the muscular bundle and both great arteries arising to the right of the ventricular septum which is best visualized in the parasternal-axis and subcostal coronal views. This view will also help determine the lack of fibrous continuity between the posterior semilunar valve and the anterior mitral valve leaflet.40)54) Echocardiographic assessment after repair should focus on ventricular septal defect patch, presence of outflow obstruction and possibility of semilunar valve regurgitation.55)
Valvular diseases that are acquired are also easily diagnosed and characterized by 2D echocardiography and Doppler or by transesophageal echocardiography. These include carcinoid heart disease, tricuspid rheumatic stenosis, pulmonary and tricuspid valve endocarditis, pulmonary and tricuspid valve insufficiency, or post-valvuloplasty pulmonary insufficiency.56)57)58)
The majority of symptoms of restrictive cardiomyopathies are due to left ventricular involvement; however, restrictive cardiomyopathies are often a global process and can affect the RV in varying degrees of hypertrophy, infiltration and abnormalities of tricuspid inflow which can be easily detected on 2D echocardiography by detecting changes in myocardial texture and on Doppler by detecting changes in flow.59)60) RV infarct can also be detected by echocardiography by evaluating the RV regional wall motion, its size, volume and global function.61)
Transthoracic echocardiography plays a role in corroborating the diagnosis of suspected pulmonary embolism and help guide management.62) The echocardiographic indices that are measured to evaluate for pulmonary embolism included RV/LV area ratio, RV/LV end diastolic dimension ratio, the McConnell sign, interventricular septal shift (D sign), pulmonary artery diameter, tricuspid regurgitation velocity, and the "60/60" sign. Of all the indices measured, RV/LV end diastolic dimension ratio > 0.7 had good accuracy for diagnosis of acute pulmonary embolism. This ratio, in addition to McConnell sign, were specific but not sensitive.63) In fact, presence of RV dysfunction, and hemodynamic instability in massive pulmonary embolism and cardiogenic shock is an indication for emergency removal of pulmonary thrombus using thrombolytics or embolectomy or catheter-based thrombus aspiration.64)
Thromboembolism and infections are a major complication associated with long term central venous catheters, port devices or pacer/implantable cardioverter defibrillator (ICD) leads. Rarely pacer/ICD leads can also migrate and cause perforations. In a retrospective study done by Chow et al.,65) 185 patients who received ICD's between 1991 and 1999 were assessed by transesophageal echocardiography at 120 ± 213 days after implantation. Forty six (25%) were diagnosed with ICD lead-related thrombi on follow-up echocardiography. Patients treated with oral anticoagulation were less likely to have thrombus formation. There was a higher incidence of lead-related thrombus in polyurethane leads than in silicone leads (35% vs. 20%; p = 0.01).
Right heart thrombi can also form in patients with inherited or acquired thrombophilia. These thrombi can be mobile or free floating or fixed. The mobile thrombi, probably originating in the peripheral veins, significantly increase the risk for pulmonary embolism. The fixed thrombi usually develop in the right heart and have a more benign prognosis. These thrombi can be detected with high sensitivity (Fig. 2D) by transesophageal echocardigraphy.66)
Right heart tumors can be non-primary or primary tumors of the heart. Non-primary tumors are more frequent and most likely the result of direct spread from adjacent malignancies or metastases from primary sites such as lung or breast cancer, lymphoma or melanoma. Primary tumors of the heart can be benign or malignant; however the benign primary tumors outnumber the malignant ones by a ratio of 3 to 1.67)68) Benign tumors of the right heart can be lipomas, myxomas, fibroelastomas or leiomyoma. Echocardiography can be helpful in distinguishing between different types of tumors based on their appearances and locations. Myxomas are often found in the left atrium. They are irregularly shaped with filamentous fronds and typically non-homogeneous.68)69)70) On the other hand, papillary fibroelastomas appear speckled with echolucencies near the edges.69) Lipomas are uncommon and may affect the atrial septum where fatty infiltration of the septum results in dramatic thickening and increased echogenicity which gives it a "dumbbell-shaped" appearance on echocardiography.67) Rhabdomyosarcomas are rare tumors.71) They appear as distinct, highly echogenic, and well-demarcated masses that often extend into the cavity of the ventricle.67) Malignant tumors of the right heart occur with less frequency and include angiosarcomas which are the commonest primary malignant tumor and rhabdomyosarcomas.67)72) Malignant tumors tend to invade or replace the myocardial tissue and alter the appearance on echocardiography. The heart often appears tethered and immobile. These tumors can be easily detected with 2D echocardiography; however, because they are less common than metastatic tumors, such echo findings should suggest the possibility of metastatic disease.73)
Pericardial effusions can be trivial and can be seen along the right atrial border on subcostal view. However, effusions can cause hemodynamic compromise by compressing the right heart structures. In fact, isolated right atrial or both right atrial and ventricular diastolic collapse and altered inflow patterns by Doppler are sensitive signs for tamponade physiology.74) Thoracic masses can also cause distortion or partial displacement or encroachment of the right heart structures as well as some degree of cavity obliteration or compression and cause tamponade physiology. In this case, the preferred method of evaluation is transesophageal echocardiography.75)
Over the past several years there has been renewed awareness about right heart and the fact that an impaired right heart function may be associated with an increased morbidity and mortality. The advantage of echocardiography over other modalities is that it is readily available, is easy to perform, is inexpensive and does not subject the patient to ionizing radiation. It is a great tool to evaluate the right heart structure and function. M-mode, 2D echocardiography with conventional Doppler and tissue Doppler as well as 3D echocardiography and it can provide significant insight into right heart physiology and pathology. As we continue to appreciate the importance of the right heart it becomes prudent to utilize echocardiography as a routine diagnostic modality to evaluate the right side of the heart in outpatient, emergency room, and inpatient settings.
Figures and Tables
Fig. 1
Echocardiographic views most frequently used to assess the structure and function of the right heart. Apical four chamber view (A). Parasternal short axis view at the level of the aortic valve showing the right atrium (RA), right ventricle (RV), right ventricular outflow tract, pulmonary valve and main pulmonary artery (B). Parasternal short axis view at the level of the papillary muscles (C). Subcostal four chamber view (D). AV: aortic valve, LA: left atrium, LV: left ventricle, PA: pulmonary artery, TV: tricuspid valve.

Fig. 2
Echocardiographic images of right heart abnormalities. Atrial septal defect in a 53-year-old woman with transient ischemic attack who declined closure (A, transesophageal echocardiography, 0 degree, arrow pointing at interatrial septum); Ebstein's anomaly in a 41-year-old man with severe tricuspid regurgitation, right atrial enlargement (B, apical four chamber view, arrow showing severely enlarged right atrium), right ventricular dysfunction and right heart failure who subsequently underwent tricuspid valve repair, right atrial reduction and maze procedure; pulmonary valve stenosis in a 38-year-old woman who had congenital pulmonic valve stenosis subsequently corrected by balloon valvuloplasty; Doppler showing high velocities across the valve (C, transesophageal echocardiography, 0 degree, arrow pointing at pulmonary valve plane); and right atrial thrombus (measuring 2 cm in length) in a 30-year-old woman who had systemic lupus erythematosus and end stage renal disease with just removed status of central venous catheter (D, transesophageal echocardiography, 105 degree, arrow pointing at the end of the pedunculated thrombus).
References
1. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010; 23:685–713. quiz 786-8.
2. Napp LC, Luesebrink U, Vogel-Claussen J, Bauersachs J, Roentgen P. Two's company: double-chambered right ventricle [corrected]. Circulation. 2013; 127:e469–e470.
3. Jurcut R, Giusca S, La Gerche A, Vasile S, Ginghina C, Voigt JU. The echocardiographic assessment of the right ventricle: what to do in 2010? Eur J Echocardiogr. 2010; 11:81–96. quiz 861-2.
4. Valsangiacomo Buechel ER, Mertens LL. Imaging the right heart: the use of integrated multimodality imaging. Eur Heart J. 2012; 33:949–960.
5. Horton KD, Meece RW, Hill JC. Assessment of the right ventricle by echocardiography: a primer for cardiac sonographers. J Am Soc Echocardiogr. 2009; 22:776–792. quiz 861-2.
6. Jaussi A, Crittin J, Bloch A. [Echocardiography of the right heart--unknown territory. Contribution of echocardiography and Doppler echocardiography to the study of the right heart]. Schweiz Rundsch Med Prax. 1989; 78:494–497.
7. Foale R, Nihoyannopoulos P, McKenna W, Kleinebenne A, Nadazdin A, Rowland E, Smith G. Echocardiographic measurement of the normal adult right ventricle. Br Heart J. 1986; 56:33–44.
8. Ho SY, Nihoyannopoulos P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart. 2006; 92:Suppl 1. i2–i13.
9. Farb A, Burke AP, Virmani R. Anatomy and pathology of the right ventricle (including acquired tricuspid and pulmonic valve disease). Cardiol Clin. 1992; 10:1–21.
10. Nesser HJ, Tkalec W, Patel AR, Masani ND, Niel J, Markt B, Pandian NG. Quantitation of right ventricular volumes and ejection fraction by three-dimensional echocardiography in patients: comparison with magnetic resonance imaging and radionuclide ventriculography. Echocardiography. 2006; 23:666–680.
11. Bleasdale RA, Frenneaux MP. Prognostic importance of right ventricular dysfunction. Heart. 2002; 88:323–324.
12. Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984; 107:526–531.
13. Pfisterer ME, Battler A, Zaret BL. Range of normal values for left and right ventricular ejection fraction at rest and during exercise assessed by radionuclide angiocardiography. Eur Heart J. 1985; 6:647–655.
14. Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001; 37:183–188.
15. Vonk MC, Sander MH, van den Hoogen FH, van Riel PL, Verheugt FW, van Dijk AP. Right ventricle Tei-index: a tool to increase the accuracy of non-invasive detection of pulmonary arterial hypertension in connective tissue diseases. Eur J Echocardiogr. 2007; 8:317–321.
16. Eidem BW, O'Leary PW, Tei C, Seward JB. Usefulness of the myocardial performance index for assessing right ventricular function in congenital heart disease. Am J Cardiol. 2000; 86:654–658.
17. Eidem BW, Tei C, O'Leary PW, Cetta F, Seward JB. Nongeometric quantitative assessment of right and left ventricular function: myocardial performance index in normal children and patients with Ebstein anomaly. J Am Soc Echocardiogr. 1998; 11:849–856.
18. Hatle L, Sutherland GR. Regional myocardial function--a new approach. Eur Heart J. 2000; 21:1337–1357.
19. Isaaz K, Munoz del Romeral L, Lee E, Schiller NB. Quantitation of the motion of the cardiac base in normal subjects by Doppler echocardiography. J Am Soc Echocardiogr. 1993; 6:166–176.
20. Yalçin F, Kaftan A, Muderrisoğlu H, Korkmaz ME, Flachskampf F, Garcia M, Thomas JD. Is Doppler tissue velocity during early left ventricular filling preload independent? Heart. 2002; 87:336–339.
21. Gondi S, Dokainish H. Right ventricular tissue Doppler and strain imaging: ready for clinical use? Echocardiography. 2007; 24:522–532.
22. Papavassiliou DP, Parks WJ, Hopkins KL, Fyfe DA. Three-dimensional echocardiographic measurement of right ventricular volume in children with congenital heart disease validated by magnetic resonance imaging. J Am Soc Echocardiogr. 1998; 11:770–777.
23. Jenkins C, Chan J, Bricknell K, Strudwick M, Marwick TH. Reproducibility of right ventricular volumes and ejection fraction using real-time three-dimensional echocardiography: comparison with cardiac MRI. Chest. 2007; 131:1844–1851.
24. Gopal AS, Chukwu EO, Iwuchukwu CJ, Katz AS, Toole RS, Schapiro W, Reichek N. Normal values of right ventricular size and function by real-time 3-dimensional echocardiography: comparison with cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2007; 20:445–455.
25. Lu X, Nadvoretskiy V, Bu L, Stolpen A, Ayres N, Pignatelli RH, Kovalchin JP, Grenier M, Klas B, Ge S. Accuracy and reproducibility of real-time three-dimensional echocardiography for assessment of right ventricular volumes and ejection fraction in children. J Am Soc Echocardiogr. 2008; 21:84–89.
26. Wang XF, Deng YB, Nanda NC, Deng J, Miller AP, Xie MX. Live three-dimensional echocardiography: imaging principles and clinical application. Echocardiography. 2003; 20:593–604.
27. Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008; 117:1436–1448.
28. Lindqvist P, Calcutteea A, Henein M. Echocardiography in the assessment of right heart function. Eur J Echocardiogr. 2008; 9:225–234.
29. Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, Schwartz T, Koch G, Clayton LM, Jöbsis MM, Crow JW, Long W. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002; 39:1214–1219.
30. Davlouros PA, Niwa K, Webb G, Gatzoulis MA. The right ventricle in congenital heart disease. Heart. 2006; 92:Suppl 1. i27–i38.
31. Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, Seward SB. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996; 9:838–847.
32. Park JH, Park MM, Farha S, Sharp J, Lundgrin E, Comhair S, Tang WH, Erzurum SC, Thomas JD. Impaired global right ventricular longitudinal strain predicts long-term adverse outcomes in patients with pulmonary arterial hypertension. J Cardiovasc Ultrasound. 2015; 23:91–99.
33. Müller H, Burri H, Lerch R. Evaluation of right atrial size in patients with atrial arrhythmias: comparison of 2D versus real time 3D echocardiography. Echocardiography. 2008; 25:617–623.
34. Kilner PJ. Imaging congenital heart disease in adults. Br J Radiol. 2011; 84 Spec No 3:S258–S268.
35. Koestenberger M. Transthoracic echocardiography in children and young adults with congenital heart disease. ISRN Pediatr. 2012; 2012:753481.
36. Rentzsch A, Abd El Rahman MY, Hui W, Helweg A, Ewert P, Gutberlet M, Lange PE, Berger F, Abdul-Khaliq H. Assessment of myocardial function of the systemic right ventricle in patients with D-transposition of the great arteries after atrial switch operation by tissue Doppler echocardiography. Z Kardiol. 2005; 94:524–531.
37. Orie JD, Anderson C, Ettedgui JA, Zuberbuhler JR, Anderson RH. Echocardiographic-morphologic correlations in tricuspid atresia. J Am Coll Cardiol. 1995; 26:750–758.
38. Alva C, Ho SY, Lincoln CR, Rigby ML, Wright A, Anderson RH. The nature of the obstructive muscular bundles in double-chambered right ventricle. J Thorac Cardiovasc Surg. 1999; 117:1180–1189.
39. Lascano ME, Schaad MS, Moodie DS, Murphy D Jr. Difficulty in diagnosing double-chambered right ventricle in adults. Am J Cardiol. 2001; 88:816–819.
40. McElhinney DB, Chatterjee KM, Reddy VM. Double-chambered right ventricle presenting in adulthood. Ann Thorac Surg. 2000; 70:124–127.
41. Geibel A. [Echocardiographic evaluation in unoperated congenital heart disease in adults]. Herz. 1999; 24:276–292.
42. Fitzgerald KP, Lim MJ. The pulmonary valve. Cardiol Clin. 2011; 29:223–227.
43. Hernandez RJ, Bank ER, Shaffer EM, Snider AR, Rosenthal A. Comparative evaluation of the pulmonary arteries in patients with right ventricular outflow tract obstructive lesions. AJR Am J Roentgenol. 1987; 148:1189–1194.
44. Yoerger DM, Marcus F, Sherrill D, Calkins H, Towbin JA, Zareba W, Picard MH. Multidisciplinary Study of Right Ventricular Dysplasia Investigators. Echocardiographic findings in patients meeting task force criteria for arrhythmogenic right ventricular dysplasia: new insights from the multidisciplinary study of right ventricular dysplasia. J Am Coll Cardiol. 2005; 45:860–865.
45. Kjærgaard J. Assessment of right ventricular systolic function by tissue Doppler echocardiography. Dan Med J. 2012; 59:B4409.
46. Apitz C, Webb GD, Redington AN. Tetralogy of Fallot. Lancet. 2009; 374:1462–1471.
47. Shinebourne EA, Babu-Narayan SV, Carvalho JS. Tetralogy of Fallot: from fetus to adult. Heart. 2006; 92:1353–1359.
48. Bleeker GB, Steendijk P, Holman ER, Yu CM, Breithardt OA, Kaandorp TA, Schalij MJ, van der Wall EE, Nihoyannopoulos P, Bax JJ. Assessing right ventricular function: the role of echocardiography and complementary technologies. Heart. 2006; 92(Suppl 1):i19–i26.
49. Dragulescu A, Grosse-Wortmann L, Fackoury C, Mertens L. Echocardiographic assessment of right ventricular volumes: a comparison of different techniques in children after surgical repair of tetralogy of Fallot. Eur Heart J Cardiovasc Imaging. 2012; 13:596–604.
50. Warnes CA. Transposition of the great arteries. Circulation. 2006; 114:2699–2709.
51. Iriart X, Horovitz A, van Geldorp IE, Barnetche T, Lederlin M, De Guillebon M, Réant P, Lafitte S, Thambo JB. The role of echocardiography in the assessment of right ventricular systolic function in patients with transposition of the great arteries and atrial redirection. Arch Cardiovasc Dis. 2012; 105:432–441.
52. George L, Waldman JD, Mathewson JW, Kirkpatrick SE, Pappelbaum SJ, Turner SW. Two-dimensional echocardiographic discrimination of normal from abnormal great artery relationships. Clin Cardiol. 1983; 6:327–332.
53. Martin RP, Qureshi SA, Ettedgui JA, Baker EJ, O'Brien BJ, Deverall PB, Yates AK, Maisey MN, Radley-Smith R, Tynan M. An evaluation of right and left ventricular function after anatomical correction and intra-atrial repair operations for complete transposition of the great arteries. Circulation. 1990; 82:808–816.
54. Hoffman P, Wójcik AW, Rózański J, Siudalska H, Jakubowska E, Włodarska EK, Kowalski M. The role of echocardiography in diagnosing double chambered right ventricle in adults. Heart. 2004; 90:789–793.
55. Choi YJ, Park SW. Characteristics of double-chambered right ventricle in adult patients. Korean J Intern Med. 2010; 25:147–153.
56. Reményi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, Lawrenson J, Maguire G, Marijon E, Mirabel M, Mocumbi AO, Mota C, Paar J, Saxena A, Scheel J, Stirling J, Viali S, Balekundri VI, Wheaton G, Zühlke L, Carapetis J. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease--an evidence-based guideline. Nat Rev Cardiol. 2012; 9:297–309.
57. Pellikka PA, Tajik AJ, Khandheria BK, Seward JB, Callahan JA, Pitot HC, Kvols LK. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation. 1993; 87:1188–1196.
58. San Román JA, Vilacosta I, López J, Revilla A, Arnold R, Sevilla T, Rollán MJ. Role of transthoracic and transesophageal echocardiography in right-sided endocarditis: one echocardiographic modality does not fit all. J Am Soc Echocardiogr. 2012; 25:807–814.
59. Berensztein CS, Piñeiro D, Marcotegui M, Brunoldi R, Blanco MV, Lerman J. Usefulness of echocardiography and doppler echocardiography in endomyocardial fibrosis. J Am Soc Echocardiogr. 2000; 13:385–392.
60. Nihoyannopoulos P, Dawson D. Restrictive cardiomyopathies. Eur J Echocardiogr. 2009; 10:iii23–iii33.
61. Esmaeilzadeh M, Parsaee M, Maleki M. The role of echocardiography in coronary artery disease and acute myocardial infarction. J Tehran Heart Cent. 2013; 8:1–13.
62. Lodato JA, Ward RP, Lang RM. Echocardiographic predictors of pulmonary embolism in patients referred for helical CT. Echocardiography. 2008; 25:584–590.
63. Leibowitz D. Role of echocardiography in the diagnosis and treatment of acute pulmonary thromboembolism. J Am Soc Echocardiogr. 2001; 14:921–926.
64. Konstantinides S. [Diagnosis and therapy of pulmonary embolism]. Vasa. 2006; 35:135–146.
65. Chow BJ, Hassan AH, Chan KL, Tang AS. Prevalence and significance of lead-related thrombi in patients with implantable cardioverter defibrillators. Am J Cardiol. 2003; 91:88–90.
66. Eweda II, Samir S, Abbas O, El-Gohary GM, Nammas W. Right heart thrombus-in-transit with pulmonary embolism in a patient with primary hypercoagulable state. Cardiol J. 2010; 17:408–411.
67. Meng Q, Lai H, Lima J, Tong W, Qian Y, Lai S. Echocardiographic and pathologic characteristics of primary cardiac tumors: a study of 149 cases. Int J Cardiol. 2002; 84:69–75.
68. Gopal AS, Stathopoulos JA, Arora N, Banerjee S, Messineo F. Differential diagnosis of intracavitary tumors obstructing the right ventricular outflow tract. J Am Soc Echocardiogr. 2001; 14:937–940.
69. Klarich KW, Enriquez-Sarano M, Gura GM, Edwards WD, Tajik AJ, Seward JB. Papillary fibroelastoma: echocardiographic characteristics for diagnosis and pathologic correlation. J Am Coll Cardiol. 1997; 30:784–790.
70. Lacey BW, Lin A. Radiologic evaluation of right ventricular outflow tract myxomas. Tex Heart Inst J. 2013; 40:68–70.
71. Sokullu O, Sanioglu S, Deniz H, Ayoglu U, Ozgen A, Bilgen F. Primary cardiac rhabdomyosarcoma of the right atrium: case report. Heart Surg Forum. 2008; 11:E117–E119.
72. Amonkar GP, Deshpande JR. Cardiac angiosarcoma. Cardiovasc Pathol. 2006; 15:57–58.
73. Labib SB, Schick EC Jr, Isner JM. Obstruction of right ventricular outflow tract caused by intracavitary metastatic disease: analysis of 14 cases. J Am Coll Cardiol. 1992; 19:1664–1668.
74. Bodson L, Bouferrache K, Vieillard-Baron A. Cardiac tamponade. Curr Opin Crit Care. 2011; 17:416–424.
75. D'Cruz IA, Feghali N, Gross CM. Echocardiographic manifestations of mediastinal masses compressing or encroaching on the heart. Echocardiography. 1994; 11:523–533.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download