A 30-year-old male presented to our emergency department with recurrent episodes of syncope for one day. Pulse was feeble with a rate of 200 per minute and systolic blood pressure was 80 mm Hg. Electrocardiogram (Fig. 1) showed a monomorphic ventricular tachycardia originating from the left ventricular outflow tract (LVOT) region (Fig. 1A). Emergency 200 J synchronized defibrillator shock was given and the rhythm reverted back to normal sinus rhythm with bifascicular block (Fig. 1B). There was no history of infective endocarditis, previous cardiac surgery or chest trauma. Cardiac auscultation revealed an early diastolic murmur at the aortic area. Chest and other systemic examination was within normal limits. Cardiac markers (troponin I and creatinine phospokinase) were negative. Chest X-ray showed cardiomegaly with multiple calcified shadows within the cardiac silhouette (white arrows, Fig. 2). Poor transthoracic acoustic window necessitated transesophageal echocardiography, which showed a huge calcified structure near the LVOT region which was compressing the left ventricuar (LV) cavity and leading to aortic regurgitation (Fig. 3, Supplementary movie 1 and 2). To further delineate the anatomy, a multidetector computed tomography was done which showed a giant (7.0 × 9.0 × 7.5 cm), calcified, multilobulated and partially thrombosed LVOT pseudoaneurysm (Fig. 4). The pseudoaneurysm had its origin in the LVOT from the mitral aortic intervalvular fibrosa region and extended anteriorly, laterally and superiorly. Invasive left ventriculogram showed a giant calcified LVOT pseudoaneurysm with only partial filling due to thrombosis within the cavity of the pseudoaneurysm (Fig. 5A and B, Supplementary movie 3 and 4). Invasive coronary angiography showed extrinsic compression (60% diameter stenosis) of the proximal left anterior descending coronary artery (Fig. 5C and D, Supplementary movie 5). Treadmill test was done which was negative for stress induced ischemia. Patient was started on amiodarone therapy and is now planned for possible surgical correction.
Pseudoaneurysms arising from LVOT region are a rare entity and exact incidence and prevalence is not known as only case reports are described in the literature. The mitral-aortic intervalvular fibrosa (MAIVF) is a fibrous triangular area in the LVOT connecting the base of the anterior mitral leaflet and the posterior aortic root.1) The relative avascular nature of MAIVF makes it susceptible to infection and injury leading to secondary pseudoaneurysm formation. Usually a prior history of either infective endocarditis, cardiac surgical intervention, chest injury or prosthetic aortic valve implantation is present.2) The patient in the present case was asymptomatic before he presented to us. The most common clinical presentation is congestive cardiac failure followed by chest pain, dyspnea and hepotysis; in some cases sudden cardiac death can be the first presenting feature.3) The pseudoaneurysm can enlarge and produce clinical features from obstruction of the surrounding structures like coronary arteries, pulmonary artery, left atrium and bronchus.4) These pseudoaneurysm also predispose to embolization and infection. Congestive cardiac failure and rupture into the surrounding structures can lead to death.4) Presentation with ventricular tachycardia, as in our case is scarcely reported. The pathophysiology of the ventricular tachycardia in the present case seem to be due to myocardial involvement per se and not due to ischemia as the cardiac markers and exercise stress test were negative for ischemia. The presence of right bundle branch block along with left axis deviation in the baseline electrocardiogram suggest erosion into the septal area by the LVOT pseudoaneurysm. Although transthoracic and transesophageal echocardiography can make a reliable diagnosis of LVOT pseudoaneurysm,5) the patient in the present case had such a huge pseudoaneurysm that it required further imaging with computed tomography and invasive LV angiography along with coronary angiography. The natural history of LVOT pseudoaneurysm is not properly know. The rate of growth of these pseudoaneurysm is also not known as no large scale echocardiographic or other imaging modality follow is available. Whether surgical correction is required in asymptomatic patients and in patients without rapid growth is debated. The surgical correction in such patients is complex often requiring aortic valve replacement, aortic root reconstruction, mitral valve repair and MAIVF reconstruction. These pseudoaneurysms are associated with high morbidity and mortality due to high risk of serious and potentially fatal complications.6) Based on this findings, most studies advice for surgical correction, without which the survival is low.6)
Figures and Tables
Fig. 1
The presenting electrocardiogram of the patient showing monomorphic ventricular tachycardia originating from the left ventricular outflow tract region (A). Post direct current cardioversion, patient regained normal sinus rhythm but with bifascicular block in the form of right bundle branch block with left anterior hemiblock (B). aVR: augmented vector right, aVL: augmented vector left, aVF: augmented vector foot.
Fig. 2
Chest X-ray showing cardiomegaly with a bulge at the upper left cardiac border along with multiple calcified round to oval shadows (white arrows) within the cardiac silhouette.
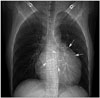
Fig. 3
Transesophageal echocardiography with color Doppler. The mid esophageal four chamber view showing a large calcified mass in the basal septal region compressing the LV cavity (A) leading to mild mitral regurgitation (B). The aortic valve was tri-leaflet as seen in the basal short axis view (C) and there was moderate aortic valve regurgitation in the LV out flow view (D). Ao: aorta, LA: left atrium, LV: left ventricle, LVOT: left ventricular outflow tract, PA: pseudoaneurysm, RA: right atrium, RV: right ventricle.
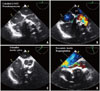
Fig. 4
Multidetector computed tomography. The communication of the pseudoaneurysm with the LVOT can be clearly seen in the axial section (A). The multilobulated nature, the calcified rims and the thrombosed lumen of the pseudoaneurysm can also be seen in the axial section (B), coronal section (C) and the sagittal section (D). Ao: aorta, LA: left atrium, LV: left ventricle, LVOT: left ventricular outflow tract, PA: pseudoaneurysm.

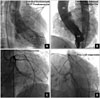
Fig. 5
Invasive left ventriculography in the right anterior oblique projection (A) and in the left anterior oblique (LAO) projection with cranial angulation (B) showing the calcified, multilobulated LVOT pseudoaneurysm filling partially due to the thrombosed internal lumen. Invasive coronary angiogram in the straight anteroposterior projection (C) and in the LAO projection with caudal angulation (D) showing the extrinsic compression of the proximal LAD coronary artery by the pseudoaneurysm. Ao: aorta, LAD: left anterior descending, LV: left ventricle, LVOT: left ventricular outflow tract.
References
1. Afridi I, Apostolidou MA, Saad RM, Zoghbi WA. Pseudoaneurysms of the mitral-aortic intervalvular fibrosa: dynamic characterization using transesophageal echocardiographic and Doppler techniques. J Am Coll Cardiol. 1995; 25:137–145.
2. Jha AK, Pandey R, Gharde P, Devagourou V, Kiran U. Idiopathic left ventricular outflow tract pseudoaneurysm. Ann Card Anaesth. 2013; 16:209–211.
3. Kaul S, Josephson MA, Tei C, Wittig JH, Millman J, Shah PM. Atypical echocardiographic and angiographic presentation of a postoperative pseudoaneurysm of the left ventricle after repair of a true aneurysm. J Am Coll Cardiol. 1983; 2:780–784.
4. Yeo TC, Malouf JF, Reeder GS, Oh JK. Clinical characteristics and outcome in postinfarction pseudoaneurysm. Am J Cardiol. 1999; 84:592–595.
5. Da Col U, Ramoni E, Di Bella I, Ragni T. An unusual left ventricular outflow pseudoaneurysm: usefulness of echocardiography and multidetector computed tomography for surgical repair. Cardiovasc Intervent Radiol. 2009; 32:188–191.
6. Savage MP, Hopkins JT, Templeton JY 3rd, Goldburgh WP, Goldberg S. Left ventricular pseudopseudoaneurysm: angiographic features and surgical treatment of impending cardiac rupture. Am Heart J. 1988; 116:864–866.
Supplementary movie legends
Movie 1
Transesophageal echocardiography. Mid esophageal four chamber view showing a huge calcified structure in the basal interventricular septal region compressin the left ventricular cavity. Also note the mildly dilated right atrium and the right ventricle.
Movie 2
Transesophageal echocardiography. Mid esophageal left ventricular outflow view showing moderate eccentric aortic regurgitation with a large thrombosed calcified structure in the left ventricular outflow region.
Movie 3
Invasive left venticulography. Right anterior oblique projection showing huge calcified and multilobulated left ventricular outflow tract psedudoaneurysm filling only partially due to thrombosed internal cavity of the pseudoaneurysm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download