Abstract
Background
After left atrial appendage (LAA) device closure, peri-device leakage into the LAA persists due to incomplete occlusion. We hypothesized that pre-procedural three-dimensional (3D) geometric analysis of the interatrial septum (IAS) and LAA orifice can predict this leakage. We investigated the predictive parameters of LAA device closure obtained from baseline cardiac computerized tomography (CT) using a novel 3D analysis system.
Methods
We conducted a retrospective study of 22 patients who underwent LAA device closure. We defined peri-device leakage as the presence of a Doppler signal inside the LAA after device deployment (group 2, n = 5) compared with patients without peri-device leakage (group 1, n = 17). Conventional parameters were measured by cardiac CT. Angles θ and φ were defined between the IAS plane and the line, linking the LAA orifice center and foramen ovale.
Results
Group 2 exhibited significantly better left atrial (LA) function than group 1 (p = 0.031). Pre-procedural θ was also larger in this group (41.9° vs. 52.3°, p = 0.019). The LAA cauliflower-type morphology was more common in group 2. Overall, the patients' LA reserve significantly decreased after the procedure (21.7 mm3 vs. 17.8 mm3, p = 0.035). However, we observed no significant interval changes in pre- and post-procedural values of θ and φ in either group (all p > 0.05).
Left atrial appendage (LAA) device closure is an alternative treatment to oral anticoagulation in patients who are unsuitable for anticoagulation therapy. The 2014 American Heart Association/American Stroke Association guidelines suggest the role of LAA device closure as an alternative strategy for stroke prevention in atrial fibrillation (AF) patients at high risk of stroke who are poor candidates for oral anticoagulation.1) In some patients, peri-device leakage of the LAA persists due to incomplete occlusion, although the procedure's success rate is generally high.2)3)4) However, information regarding the presence of and factors contributing to peri-device leakage is scarce, and further study to evaluate the anatomical and procedure-related factors of this leakage has not been performed. Left atrium (LA) and LAA geometry is complex; therefore, only limited data are available for predicting procedural success based on cross-sectional imaging surrogates. The relative location of the LAA orifice to the interatrial septum (IAS) varies, and these differences may affect the success rate of device implantation.5)
We hypothesized that three-dimensional (3D) anatomical imaging of the relative locations of the IAS and LAA orifice could predict peri-device leakage after LAA device closure. However, no data exist on the outcome after LAA device closure linking these anatomical relationships. We also hypothesized that reverse remodeling of LA and functional recovery after closure could impact the occurrence of post-procedural complication.
Therefore, we aimed to investigate the predictive parameters of LAA device closure obtained from baseline cardiac computerized tomography (CT) imaging using a novel 3D analysis system. We also explored if changes in cardiac geometry and function after LAA device closure correlate with procedural success.
We conducted a retrospective, observational study. Thirty-one patients who had undergone LAA device closure at Severance Cardiovascular Hospital, Yonsei University, were consecutively enrolled in the Yonsei LAA Occluder Registry from October 2010 to April 2014. Among them, nine patients who had not undergone pre- and post-procedural cardiac CT imaging were excluded. Thus, 22 patients were enrolled in this study, 14 of which received a WATCHMAN device (Boston Scientific, Natick, MA, USA) and 8 of which received Amplatzer cardiac plugs (ACPs) device (St. Jude Medical, Menneapolis, MN, USA) (Fig. 1). The clinical, procedural, and echocardiographic outcome variables of these patients were retrospectively reviewed and analyzed. All patients had AF with high risk for stroke (CHADS2 score ≥ 2 or CHA2DS2-VASc score ≥2)6)7) and bleeding or contraindication to anticoagulation. Exclusion criteria for LAA device closure were as follows: 1) LAA thrombus or 2) active endocarditis.
Traditional or novel oral anticoagulation therapy was continued in the patients with the WATCHMAN device for 3 months post-procedure. Anticoagulation medication was discontinued at time of discharge in patients with ACP. Procedural success was defined as successful implantation of the device in the LAA without any complication, such as pericardial effusion, tamponade, peri-device leakage, or stroke. The study protocol was approved by the Institutional Review Board of Severance Cardiovascular Hospital, Seoul, Korea and complied with the Declaration of Helsinki, and written informed consents were obtained from all study participants.
All patients underwent pre-procedural transesophageal echocardiography (TEE) with a Philips iE33 machine (Philips Medical System, Andover, MA, USA) equipped with a multiplane probe X7-2t to detect possible LAA thrombi and measure the LAA orifice diameter as described previously.8) Two-dimensional-TEE images were obtained every 30°. Based on these images, a WATCHMAN device 10-20% larger than the LAA diameter and lobe sizes of ACP 3-5 mm larger than the landing zone diameter were selected to ensure stable device positioning.9)10) We also measured the LAA orifice diameter and landing zone using cardiac CT as described at the next paragraph. In the case of different measurements between preprocedural TEE and cardiac CT, the largest size was chosen. The procedure was performed under general anesthesia, and TEE was performed during the full procedure. After transseptal puncture, a delivery catheter was advanced to approach the LAA, and the selected device was implanted. Device placement into the LAA was perpendicular to the LAA landing zone axis (ACP), or the device was advanced until both of its distal marker bands and access sheath were aligned (WATCHMAN). Intravenous heparin was administered to maintain an activated clotting time of > 250 sec until completion of the procedure.
Follow-up transthoracic echocardiography was performed within 1 day to detect device embolization or pericardial effusion after the procedure. Follow-up TEE was performed 2 or 3 months after the procedure to monitor the presence of residual peri-device flow between the LAA and LA, detect thrombi on the device, and determine device stability and positioning. Presence of a Doppler signal inside the LAA after device deployment indicated leakage (Fig. 2). The severity of peri-device flow was defined as minor (jet width < 1 mm), moderate (1 mm to 3 mm), or major (> 3 mm).
All patients underwent pre-procedural cardiac CT to measure the LAA orifice diameter and evaluate the LAA morphology. Cardiac CT of each patient was performed with multiple-detector CT (320-detector CT, Aquilion®; Toshiba Medical System Corporation, Otawara, Japan), and images were analyzed using commercially available post-processing software (Vitrea®, Vital Images, Toshiba Medical System Corporation, Otawara, Japan). We measured LA volume, ostium diameter, and orifice area and subsequently calculated LA reserve and function. Maximal and minimal LA and LAA volumes were measured with acquired images at 30% and 70% of the R-R interval, respectively, from which LA reserve and LA/LAA function was calculated. The reserve was determined by subtracting the minimal volume from the maximal volume, and function was expressed as a percentage of the reserve calculation.
The LAA morphology of each patient was classified into one of four groups as described previously:11)12) 1) wind sock: one dominant lobe of sufficient length plus secondary or tertiary lobes arising from the dominant lobe, 2) chicken wing: a sharp bend in the proximal or medial part of the dominant lobe at some distance from the perceived LAA ostium, 3) cactus: a dominant central lobe with small chambers extending in all directions, or 4) cauliflower: complex internal characteristics with no dominant lobe.
Follow-up cardiac CT was also performed 2 or 3 months after the procedure to evaluate the change of LA/LAA function, reserve and geometry.
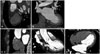
We developed a 3D software system to measure the angle between the IAS and LAA orifice based on Visual Studio C++ 2008 (Microsoft, Seattle, WA, USA). We defined one plane (IAS plane) with three points (P, A, and U) to measure the angles (θ, φ) between the IAS and LAA with a 3D approach (Fig. 3). At first, the point L was chosen at the center of the LAA orifice (Fig. 4A and B). P is the foramen ovale, which is usually assessed as an IAS puncture site (Fig. 4C, D, and E), and A is defined as the point at which the aortic root and IAS plane are aligned in oblique view (Fig. 4D and E). U is an arbitrary point that lies on the IAS (Fig. 4D). All points were manually selected in oblique view. We defined a⃗ as the vector from P to A, and u⃗ as the vector from P to U. The plane n⃗, the normal vector which represents the IAS plane, is given as the outer product of a⃗ and u⃗, which can be written as a⃗ × u⃗ = n⃗. Hence, the vector l⃗ = L - P can be specified with two parameters, θ and φ, in the spherical coordinate system. Both θ and φ can be measured as the inner products of n⃗·l⃗ and a⃗·l⃗' respectively, and l⃗' is the projected vector l⃗ onto IAS plane (Fig. 3). θ is the angle between vector n⃗ and l⃗, and φ is the angle between vector a⃗ and the projected vector l⃗ onto IAS plane. Then, two angles were defined: 1) θ, the angle between the IAS plane and the line, linking the LAA orifice center and the foramen ovale, and 2) φ, the angle between the IAS and the LAA orifice center. These two values were calculated with four coordinate values using the new software. We defined another coordinate system (a, y, n) from the given points (P, U, and A), and suggested the formulas to calculate θ and φ as described (Supplementary Fig. 1). Total time required to measure and calculate these two angles per one study was within 10 minutes.
Continuous variables are presented as the median (25th, 75th percentiles) due to the non-parametric model, and categorical variables are presented as numbers (%). For comparison of continuous variables among groups, a Kruskall-Wallis test was performed. The chi-square test was performed for comparing categorical variables. Analysis was performed with the statistical software SPSS 20.0 for Windows (IBM, Markham, Canada). A two sided p-value of < 0.05 indicated statistical significance.
Of the 22 study participants, 16 patients (31.8%) were women, and the median age of the study group was 63 (54, 71) years. Baseline characteristics are presented in Table 1. Severe peri-device leakage occurred in 5 patients (22.7%), either immediately after device implantation or during the follow-up period. Three patients (13.6%) experienced intraprocedural peri-device leakage, and 2 subjects (9.1%) without intraprocedural leakage showed newly developed leakage at their 2-3 month follow-up. Patients were divided into two groups: group 1, without peri-device leakage (n = 17) and group 2, with peri-device leakage (n = 5). We observed no significant differences in the presence of hypertension, diabetes, age, and gender between the two groups. Conventional echocardiographic parameters such as left ventricular ejection fraction, LA volume index, and LAA emptying velocity were similar between the two groups.
The device was implanted in all 22 patients without embolization, and immediate procedural success rate was 77.3% (17/22). Two patients developed peri-procedural pericardial effusion without cardiac tamponade, and two patients experienced post-procedural stroke. Warfarin was discontinued in ten WATCHMAN patients (10/14, 71.4%) after a 2-3 month follow-up TEE and in six ACP patients (6/8, 75.0%) at discharge. In patients without peri-device leakage, two patients continued taking warfarin because of renal infarction (1 patient) or post-procedural stroke (1 patient).
Pre-procedural cardiac CT findings are presented in Table 2. We observed no significant difference in LAA orifice diameter or LAA volume and function between the two groups. However, LA function was better in group 2 than group 1 [11.3% (6.3, 13.7) vs. 17.0% (14.7, 20.9), p = 0.031]. In addition, preprocedural θ was larger in group 2 [41.9° (37.6, 41.9) vs. 52.3° (44.2, 58.8), p = 0.019]. Cauliflower-type morphology was more common in group 2 (80.0% vs. 35.3%), but the chicken wing type was more common in group 1 (52.9% vs. 20.0%).
Post-procedural cardiac CT findings are shown in Table 3. We saw no significant difference in LA volume or function between group 1 and group 2. We also observed no significant interval change between pre- and post-procedural LA function [12.4% (7.5, 15.4) vs. 9.9% (4.3, 12.7), p = 0.135], but LA reserve significantly decreased after the procedure [21.7 mm3 (12.2, 30.0) vs. 17.8 mm3 (8.4, 26.2), p = 0.035]. In contrast, we saw no significant difference between groups 1 and 2 with respect to post-procedural θ and φ or pre- and post-procedural interval changes (all p > 0.05).
Implanting an LAA device by making a separate transseptal puncture in the inferoposterior direction is generally preferred to provide more direct vector orientation to access the LAA.13)14) However, using 3D geometry characterization to predict peridevice leakage has not been well studied. Here, we developed a novel mathematical approach to accurately measure the 3D relationship between the IAS and LAA orifice planes. We found that angulation between the planes significantly affected procedural results, such as development of peri-device leakage. Thus, we propose pre-procedural individualized catheter selection, manual shaping of the puncture needle, and/or adjustment of the IAS puncture angle according to pre-operative 3D anatomical characterization to limit these post-operative complications.
Geometric complexity, individual variation in LAA morphology, or mismatch between the device shape and LAA morphology may result in incomplete occlusion with a gap between the device and LAA, causing peri-device leakage.4)11)15)16) The device is implanted via a transseptal route into the LAA orifice, and the resulting angle might be related to device stability, positioning, and possible leakage. However, current imaging methods cannot accurately measure this angle. Therefore, we designed a new program to measure the angle using one plane and one point and evaluated the anatomical relationship of the IAS and LAA orifice, which could significantly impact complete LAA device occlusion.
Among the four LAA morphological types, the cauliflower type is most often associated with peri-device leakage and an embolic event in AF patients. These patients exhibit complex internal characteristics, a variable number of lobes without a dominant lobe, and a more irregular shape of the orifice.12) Cardiac CT with 3D imaging can provide morphological classification associated with high-risk peri-procedural complications. However, morphological classification alone cannot predict periprocedural success in every dynamic intra-cardiac catheter-related procedure. With this 3D geometric analysis, we can also evaluate the relationship between IAS and imaginary LAA orifice planes and LAA long axis alignment. This analysis is important because the LAA orifice plane is not always perpendicular to the catheter's direction. Without perpendicularly introducing the device mounted on a catheter tip, safe and optimal deployment in the LAA is difficult. Application of this novel 3D analysis program could be extended to many peri-procedural situations. The program is easily and conveniently applied via most commercial analysis workstations commonly used for clinical practice and research purposes.
Several devices are now used or tested with respect to LAA occlusion. Although technical aspects of delivery differ between devices, transseptal puncture with a sheath is required to facilitate delivery into or around the LAA.17) Typically, the sheath is delivered under the guidance of TEE and fluoroscopy. However, the angle between the IAS and LAA orifice differs by individual. Moreover, 3D anatomical consideration is not currently possible with conventional methods of device placement. We found that the angle between the IAS plane and the line linking the LAA orifice center and foramen ovale was significantly different between patients with or without peri-device leakage. A larger angle was observed in patients with peri-device leakage, suggesting that the additive effects of natural angulation with the direction of the advancing puncture needle and catheter are related to anatomical mismatch between the LAA and the device. Our novel analysis can provide valuable individualized pre-operative information to predict post-operative outcomes.
Several reports suggest peri-device leakage after LAA closure is benign,4)18)19) but this complication could lead to a negative clinical outcome. In the WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation (PROTECT-AF) substudy, Viles-Gonzalez et al.4) demonstrated that residual peri-device leakage after LAA closure with a WATCHMAN device was not associated with risk of thromboembolic events or increased cardioembolic risk. Urena et al.3) also reported that mild peri-device leakage was not associated with thromboembolic events after LAA closure with ACP. However, these results may not provide sufficient evidence because of the studies' relatively short follow-up duration and low event rate that limited the power of the analyses. Long-term follow-up is necessary to demonstrate the effect of peri-device leakage on clinical outcomes such as cardioembolic events, and complete LAA closure should be attempted. Further large cohort study should be needed to clarify the predictors for the successful result of LAA device closure. Prospective studies to evaluate not only the relation between 3D geometry and peri-device leakage, but also the clinical outcome according to the LA and LAA geometry might be needed. Furthermore, the prospective study with individualized catheter selection, manual shaping of puncture needle or device selection may enable the tailored procedure according to their anatomy, which might improve the clinical outcome.
Another previous study showed that peri-device leakage was not detected at immediate post-procedural status but was observed at the 6-month follow-up TEE,3) indicating different mechanisms may exist for earlier and later occurrences of peridevice leakage. Late occurrence could be due to an under-sized device, incomplete device compression, or incomplete endothelization around the device,19)20) whereas earlier occurrence may be associated with anatomical mismatch. In our study, peridevice leakage in most patients was detected during both periprocedural and follow-up TEE, which ruled out possible periprocedural residual flow due to LAA contraction. We conclude that peri-device leakage of our enrolled patients resulted from their individual anatomical geometry. Thus, our study is the first to suggest anatomical location and internal characteristics of the LA and LAA might affect both immediate and delayed peri-device leakage.
Volume and function of the LA are crucial because they are linked to diastolic function and post-operative cardiovascular outcomes.21)22)23)24)25) Some studies have shown that LAA exclusion may alter LA compliance, function, or neurohormonal status because LAA is a source of atrial natriuretic peptide. However, in our study, LAA occlusion altered LA reserve, highlighting the need for further investigation incorporating neurohormonal markers and 3D-analyzed LA function and geometry. Our novel 3D geometrical analysis program could certainly contribute to this analysis.
The present study has several limitations. First, subjects were selected from a single-center registry database. The small sample size led to a low number of peri-device leakage events, and lack of long-term follow-up data made predicting clinical outcomes difficult. Secondary, we included patients who underwent either ACP or WATCHMAN device implantation, which might affect the incidence of peri-device leakage independently of different patient anatomical characteristics. Thirdly, although we measured geometric parameters using foramen ovale, septal puncture site is not guaranteed to match to foramen ovale center in actual procedure. Fourthly, we measured the LA volume with acquired images at 30% and 70% phase of cardiac cycle, which would not matched to systole and diastole of LA in AF patients.
Morphology of the LA and angles between the IAS and LAA orifice measured by 3D CT might be novel anatomical parameters for predicting peri-device leakage after LAA device closure and could identify favorable candidates for the procedure. Furthermore, individualizing the procedure based on the patient's own anatomical geometry could significantly reduce the occurrence of some post-operative cardiovascular complications.
Figures and Tables
Fig. 2
Peri-device leakage identified by transesophageal echocardiography (TEE) color Doppler imaging. The TEE image shows incomplete device sealing with residual flow of 4 mm. The average flow rate was 10 cm/sec.
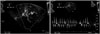
Fig. 3
A spherical coordinate system illustrates three dimensional image space where the vector direction of the LAA center is specified by two numbers. To measure the angles (θ, φ) between the IAS and LAA with a 3D approach, we defined one plane (IAS plane) with three points: the foramen ovale (P), an arbitrary point on IAS plane (U), a defined point at the intersection of aortic root and IAS plane (A) and the center of LAA orifice (L). LAA: left atrial appendage, IAS: interatrial septum, 3D: three-dimensional.
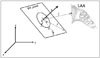
Fig. 4
The program was developed to measure the angle between the interatrial septum and left atrial appendage (LAA) orifice. In a real CT volume image, four points were defined to calculate the angle between the interatrial septum and LAA orifice: the center of LAA orifice (the point with thick arrows at A and B), the foramen ovale (the point with thick arrows at C, D, and E), an arbitrary point (the point with short thin arrow at D) and a defined point (point with long thin arrows in D and E).
Acknowledgements
This work was supported by Institute for Information & communications Technology Promotion (IITP) grant funded by the Korea government (MSIP) (No.R0101-15-0171, Development of Multi-modality Imaging and 3D Simulation-Based Integrative Diagnosis-Treatment Support Software System for Cardiovascular Diseases).
References
1. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA. American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014; 45:2160–2236.
2. Reddy VY, Doshi SK, Sievert H, Buchbinder M, Neuzil P, Huber K, Halperin JL, Holmes D. PROTECT AF Investigators. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (Watchman left atrial appendage system for embolic protection in patients with atrial fibrillation) trial. Circulation. 2013; 127:720–729.
3. Urena M, Rodés-Cabau J, Freixa X, Saw J, Webb JG, Freeman M, Horlick E, Osten M, Chan A, Marquis JF, Champagne J, Ibrahim R. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol. 2013; 62:96–102.
4. Viles-Gonzalez JF, Kar S, Douglas P, Dukkipati S, Feldman T, Horton R, Holmes D, Reddy VY. The clinical impact of incomplete left atrial appendage closure with the Watchman device in patients with atrial fibrillation: a PROTECT AF (percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation) substudy. J Am Coll Cardiol. 2012; 59:923–929.
5. Fender EA, Sibley CT, Nazarian S, Cheng A, Spragg DD, Marine JE, Berger RD, Calkins H, Lima JA, Brinker JA, Henrikson CA. Atrial septal angulation varies widely in patients undergoing pulmonary vein isolation. J Invasive Cardiol. 2014; 26:128–131.
6. Gage BF, van Walraven C, Pearce L, Hart RG, Koudstaal PJ, Boode BS, Petersen P. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004; 110:2287–2292.
7. Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010; 41:2731–2738.
8. Veinot JP, Harrity PJ, Gentile F, Khandheria BK, Bailey KR, Eickholt JT, Seward JB, Tajik AJ, Edwards WD. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. 1997; 96:3112–3115.
9. Aryana A, Saad EB, d'Avila A. Left atrial appendage occlusion and ligation devices: what is available, how to implement them, and how to manage and avoid complications. Curr Treat Options Cardiovasc Med. 2012; 14:503–519.
10. De Backer O, Arnous S, Ihlemann N, Vejlstrup N, Jørgensen E, Pehrson S, Krieger TD, Meier P, Søndergaard L, Franzen OW. Percutaneous left atrial appendage occlusion for stroke prevention in atrial fibrillation: an update. Open Heart. 2014; 1:e000020.
11. Wang Y, Di Biase L, Horton RP, Nguyen T, Morhanty P, Natale A. Left atrial appendage studied by computed tomography to help planning for appendage closure device placement. J Cardiovasc Electrophysiol. 2010; 21:973–982.
12. Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, Horton R, Sanchez JE, Bai R, Mohanty S, Pump A, Cereceda Brantes M, Gallinghouse GJ, Burkhardt JD, Cesarani F, Scaglione M, Natale A, Gaita F. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012; 60:531–538.
13. Ho IC, Neuzil P, Mraz T, Beldova Z, Gross D, Formanek P, Taborsky M, Niederle P, Ruskin JN, Reddy VY. Use of intracardiac echocardiography to guide implantation of a left atrial appendage occlusion device (PLAATO). Heart Rhythm. 2007; 4:567–571.
14. Mráz T, Neuzil P, Mandysová E, Niederle P, Reddy VY. Role of echocardiography in percutaneous occlusion of the left atrial appendage. Echocardiography. 2007; 24:401–404.
15. Blendea D, Heist EK, Danik SB, Barrett C, Ruskin JN, Mansour M. Analysis of the left atrial appendage morphology by intracardiac echocardiography in patients with atrial fibrillation. J Interv Card Electrophysiol. 2011; 31:191–196.
16. Bai R, Horton RP, DI Biase L, Mohanty P, Pump A, Cardinal D, Scallon C, Mohanty S, Santangeli P, Brantes MC, Sanchez J, Burkhardt JD, Zagrodzky JD, Gallinghouse GJ, Natale A. Intraprocedural and long-term incomplete occlusion of the left atrial appendage following placement of the WATCHMAN device: a single center experience. J Cardiovasc Electrophysiol. 2012; 23:455–461.
17. Merchant FM, Delurgio DB. Site-specific transseptal cardiac catheterization guided by intracardiac echocardiography for emerging electrophysiology applications. J Innov Cardiac Rhythm Manage. 2013; 4:1415–1427.
18. Bayard YL, Omran H, Neuzil P, Thuesen L, Pichler M, Rowland E, Ramondo A, Ruzyllo W, Budts W, Montalescot G, Brugada P, Serruys PW, Vahanian A, Piéchaud JF, Bartorelli A, Marco J, Probst P, Kuck KH, Ostermayer SH, Büscheck F, Fischer E, Leetz M, Sievert H. PLAATO (percutaneous left atrial appendage transcatheter occlusion) for prevention of cardioembolic stroke in non-anticoagulation eligible atrial fibrillation patients: results from the European PLAATO study. EuroIntervention. 2010; 6:220–226.
19. Freixa X, Tzikas A, Sobrino A, Chan J, Basmadjian AJ, Ibrahim R. Left atrial appendage closure with the Amplatzer™ Cardiac Plug: impact of shape and device sizing on follow-up leaks. Int J Cardiol. 2013; 168:1023–1027.
20. Neuzner J, Dietze T, Paliege R, Möller M, Saeed G, Gradaus R. Left atrial appendage closure with the Amplatzer™ Cardiac Plug: Rationale for a higher degree of device oversizing at implantation. Cardiol J. 2015; 22:201–205.
21. Otani K, Takeuchi M, Kaku K, Haruki N, Yoshitani H, Tamura M, Abe H, Okazaki M, Ota T, Lang RM, Otsuji Y. Impact of diastolic dysfunction grade on left atrial mechanics assessed by two-dimensional speckle tracking echocardiography. J Am Soc Echocardiogr. 2010; 23:961–967.
22. Wong RC, Yeo TC. Left atrial volume is an independent predictor of exercise capacity in patients with isolated left ventricular diastolic dysfunction. Int J Cardiol. 2010; 144:425–427.
23. Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, Roman MJ, Devereux RB. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS). Am Heart J. 2006; 151:412–418.
24. Tsang TS, Barnes ME, Gersh BJ, Takemoto Y, Rosales AG, Bailey KR, Seward JB. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol. 2003; 42:1199–1205.
25. Chinali M, de Simone G, Roman MJ, Bella JN, Liu JE, Lee ET, Best LG, Howard BV, Devereux RB. Left atrial systolic force and cardiovascular outcome. The Strong Heart Study. Am J Hypertens. 2005; 18(12 Pt 1):1570–1576.
Supplementary Material
The online-only Data Supplement is available with this article at http://dx.doi.org/10.4250/jcu.2015.23.4.211.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download