Introduction
Stress induced cardiomyopathy (SCM) is characterized by transient left ventricular (LV) dysfunction and chest pain, together with electrocardiographic (ECG) changes that mimic those of acute myocardial infarction (AMI). Generally, it has been known that in the absence of underlying comorbid conditions, SCM has good prognosis. We experienced a rare case of SCM associated with ventricular septal rupture (VSR)
Case
An 87-year-old woman with continuous chest discomfort was referred to our hospital and we suspected that she had AMI. She slipped down on the floor and was admitted to another hospital due to multiple rib fractures and stayed there for 10 days. On admission to our hospital, she complained of chest pain. She had no history of cardiovascular risk factors nor did she have family history of heart disease. Her blood pressure was 80/40 mm Hg and her pulse rate was 125 beats/min. Systolic murmur was heard through cardiac auscultation. An ECG was done on admission and sinus rhythm, ST-segment elevation and abnormal Q wave in leads V1-4 were observed (Fig. 1A).
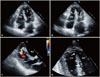
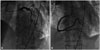
Chest radiography showed no signs of cardiomegaly, nor signs of pulmonary congestion. Laboratory data on admission showed increased levels of cardiac biomarkers (troponin-I 8.2 ng/nL and CK-MB 50 U/L). Echocardiography showed akinesis in apical segments of LV and right ventricular (RV), and hyperkinesis in the basal segments of LV (Fig. 2A and B). Filling defect (0.8 cm in size) and left to right shunt flow were shown on apical segment of interventricular septum (Fig. 2C and D). Post-AMI VSR was suspected and she underwent emergency cardiac catheterization. However, coronary angiography revealed normal coronary artery with thrombolysis in myocardial infarction grade 3 (Fig. 3). Angiographic findings showed no spontaneous coronary spasm, nor thrombus. However, we could not perform coronary spasm provocation test. Since the patient and her family did not want to get a cardiac operation, only medical therapy including intravenous inotropics was continued. Her peak troponin-I level gradually decreased during follow-up examinations. On the third day after admission, ECG showed persistent ST-segment elevation in V2-5, and Q-wave in lead aVR, III, and V1-4 (Fig. 1B). On the fourth day after admission, polymorphic ventricular tachycardia developed (Fig. 1C), and the patient died even after cardiopulmonary resuscitation was performed (Fig. 1D).
Discussion
Patients suffering from SCM may have clinical presentation very similar to that of acute coronary syndrome patients. Indeed, most patients present chest pain or dyspnea. Usually, ECG at presentation shows ST-segment elevation and/or pathological Q waves are present in almost 40% of patients.1)2) Furthermore, elevated cardiac biomarker levels and regional wall motion abnormalities mimic AMI. Differentiating SCM from AMI is important, as misdiagnosis may result in treatment with thrombolytic agents which may pose patients at unnecessary risk of bleeding. Clinical features of SCM are as follows: 1) abnormal, reversible wall motion that improves rapidly within a few weeks, 2) abnormal ECG patterns (either ST-segment elevation or T-inversion) or slightly elevated cardiac biomarker levels, 3) no significant epicardial coronary artery stenosis nor vasospasm, 4) presence of physical or emotional stress as trigger factors, 5) absence of recent significant head trauma, intracranial bleeding, pheochromocytoma, or myocarditis.3)4)
Reported pathologic findings of cardiac rupture in SCM are inflammatory infiltration, coagulation necrosis, hemorrhage, fibrosis, and contraction band necrosis.5) These findings imply that prolonged ischemia causes cardiac rupture. Contraction band necrosis is a kind of myocardial injury, but it is different from ischemic cardiac necrosis.6) Sacha et al.7) reported that contraction band necrosis at free wall rupture site is a typical result of catecholamine cardiotoxicity and that it may play a primary role in cardiac rupture. In SCM patients, cardiac rupture can occur at certain sites of LV, such as interventricular septum, RV wall, and LV free wall. Izumi et al.8) reported a case of ventricular septal perforation in a SCM patient. The case was very similar to ours in that the patient was an old female, and had persistent ST-segment elevation along with slightly elevated cardiac enzyme levels.
We could not confirm reversible wall motion abnormalities in this case, but based on the following findings (ST-segment elevation without reciprocal changes on ECG, normal coronary angiogram, physical stress as a trigger factor, characteristic abnormal wall motion on echocardiography, and moderately elevated cardiac biomarker), we suspected that this patient had SCM associated with VSR. Coronary spasm and thrombus can be the cause of post-AMI VSR in normal coronary artery. However, since the patient had no history of angina nor cardiovascular risk factors except for her old age, there is low possibility of her having had variant angina or thrombus and thus, there is low chance that her VSR was developed by AMI. Therefore, we suspected that her VSR was developed by SCM, due to tissue necrosis from catecholamine toxicity.
The prognosis of patients with SCM is generally favorable.9) However, some fatal complications with SCM, such as LV free wall rupture, were reported. SCM can cause cardiac rupture in a manner and time course similar to that of AMI. However, there are some differences between AMI induced cardiac rupture and SCM induced cardiac rupture.8) The diagnosis of cardiac rupture caused by SCM, not by AMI should be considered in the presence of emotional or physical trigger factors, abnormal wall motion which is not consistent with coronary territory, cardiac enzyme elevation not to correlated infarct size of LV, persistent ST-segment elevation even after continued medical treatment, and the finding of normal coronary angiogram.
In conclusion, we highly suspect that VSR was developed by SCM although coronary vasospasm could not be thoroughly excluded.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download