Abstract
Cardiac involvement is a major cause of morbidity and mortality in hypereosinophilic syndrome (HES). It is classified into 3 stages by the degree of eosinophils-mediated heart injury; acute necrotic stage, thrombotic stage, and fibrotic stage. Nonetheless, definitive evidence that each patient passes sequentially through these stages is lacking. We present a case of 48-year-old male patient with dyspnea and peripheral edema who underwent valve replacement surgery due to severe mitral regurgitation. After the valve replacement, HES with cardiac involvement in the thrombotic stage was diagnosed. In the follow-up study, the patient progressed into fibrotic stage of HES.
The hypereosinophilic syndrome (HES) is a group of disorders characterized by overproduction of eosinophils that cause involvement in multiple organs including heart. Definition of HES includes persistent eosinophilia (> 1500/mm3) in the absence of other apparent causes of eosinophilia such as parasitic or allergic disease, with the evidence of eosinophil-mediated end organ damage.1) HES presents with various symptoms and signs; dermatologic (e.g., rash), pulmonary such as cough and dyspnea, gastrointestinal and cardiovascular.2) Cardiac involvement is frequent and can be a major cause of morbidity and mortality in HES.3) It is characterized by endocardial involvement with thrombus formation, endocardial fibrous thickening and apical obliteration. Sometimes, it is not easily diagnosed until the pathologic study shows the characteristic features of HES. Therefore, sequential changes in each patient are rarely reported.3) We report a case of HES with cardiac involvement progressing from thrombotic to fibrotic stages in more than 4 years' follow-up.
A 48-year-old man presented with New York Heart Association class III dyspnea and pitting edema in both legs for 2 months. The initial chest X-ray showed increased cardiothoracic ratio with pulmonary congestion and electrocardiography demonstrated sinus tachycardia with left atrial enlargement and left anterior fascicular block. Transthoracic echocardiography (TTE) showed severe mitral regurgitation (MR) with coaptation failure and mild endocardial thickening in the apex and lateral wall of left ventricle (Fig. 1). He underwent mitral valve replacement with tissue valve and tricuspid annuloplasty. During the operation, there were multiple dot lesions at left ventricular myocardium, which was proved to be organized thrombi by pathologic examination (Fig. 2). After the operation he was lost to follow-up.
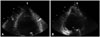
Two years after operation, he visited cardiology clinic and wanted to know the valve state. Follow-up echocardiography showed normally functioning tissue valve and a homogeneous fibrotic obliteration of the left ventricular apex (Fig. 3). The laboratory test showed an increased eosinophil count of 616/mm3 (9.4% of leukocytes) and HES was suspected. The bone marrow study showed normocellular marrow with mildly increased eosinophils and there was no evidence of malignancy. Parasite test was negative. Although eosinophil count did not meet with the diagnostic criteria of HES, review of medical records revealed that the maximum eosinophil count was 1948/mm3 (25.8% of leukocytes) when the patient underwent valve replacement surgery. He was diagnosed as HES due to the typical echocardiographic findings of HES and a supporting evidence of eosinophilia of more than 1500/mm3 in the previous laboratory test. We recommended steroid therapy. However he refused to use steroid therapy and was lost to follow-up again.
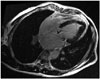
Another two years later, he revisited. The prosthetic valve was intact but the obliteration in the left ventricular apex became more prominent. The cardiac magnetic resonance imaging demonstrated a diffuse subendocardial delayed enhancement at the apex (Fig. 4), which was compatible with fibrosis. Corticosteroid was prescribed. Unfortunately, however, osteonecrosis of the left femoral head occurred after 9 months and steroid was stopped. He is now only on warfarin.
This report is of a clinical importance as serial TTE demonstrates both thrombotic and fibrotic stages of cardiac involvement with HES more than 4 years. HES should have been suspected initially. However clinical urgency of severe symptomatic MR seems to put the patient into operative intervention first. And unfortunately unusual operative findings were missed and the patient was lost to follow-up.
Cardiac involvement is major cause of morbidity and mortality in HES. The spectrum of cardiac involvement is broad, ranging from necrosis to thrombosis or fibrosis. Eosinophil-mediated cardiac injury is classified into three stages.4) During the early period of the disease, eosinophils and lymphocytes infiltrate the myocardium and hypersensitivity reaction occur and this is known as the acute necrotic stage. There are no typical symptoms or abnormal echocardiographic finding at this stage and endocardial biopsy may be needed to make a diagnosis. During the thrombotic stage, endomyocardial and valvular involvement of HES can be occurred and thrombus formation can be accompanied. Finally at the fibrotic stage, the endomyocardial fibrosis and scarring progresses and restrictive cardiomyopathy can be followed.4)5)
To evaluate HES with cardiac involvement, echocardiography is the most useful non-invasive diagnostic tool that can be easily implemented. The typical findings are endomyocardial thickening, thrombus formation at ventricular apex and mitral leaflet involvement. Subvalvular damage can aggravate the regurgitation of the atrioventricular valves.3) According to U.S. National Institute of Health study of HES patients, characteristic echocardiographic features are left ventricular free wall thickening (68%), left atrial transverse dimension increasing (37%), right ventricular transverse dimension increasing (27%) and pericardial effusion (32%).3)5) The most distinct feature among previously described echocardiographic findings is the obliteration of the apex in the left or right ventricle by laminar thrombus.6)
HES is an unusual cause of acute MR. In this case, the formation of mural thrombus might be involved in subvalvular apparatus and it made atrioventricular valve incompetence.7) Surgical experience in these cases is very limited. Valve replacement was performed in most cases. As replacement with mechanical valve was frequently accompanied with thrombotic complication, bioprosthesis is recommended.3)8)
The clinical importance of this case report is the serial TTE during follow-up demonstrated both thrombotic and fibrotic stages of cardiac involvement with HES for more than 4 years' duration. Initial TTE demonstrated severe MR without definite valve pathology such as prolapse or chordae rupture and mild endocardial thickening in lateral wall and apex of left ventricle. Moreover there were eosinophilia; the highest eosinophil count was about 1948/mm3. It had better to consider HES with cardiac involvement at the first visit. With the serial follow-up of TTE we were able to find the homogeneous fibrotic obliteration at the left ventricle apex which represents endomyocardial fibrosis, the typical feature observed during the chronic fibrotic stage.
Figures and Tables
Fig. 1
Preoperative echocardiography shows mild endocardial thickening in lateral wall and apex of left ventricle (A) with severe mitral regurgitation and moderate tricuspid regurgitation (B).
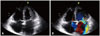
Fig. 2
Histopathologic analysis of left ventricle shows fibrin clot with blood clot, inflammatory cells (A) and eosinophil infiltration (B) (hematoxylin and eosin stain, × 200).

References
1. Chusid MJ, Dale DC, West BC, Wolff SM. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore). 1975; 54:1–27.
2. Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, Leiferman KM, Nutman TB, Pfab F, Ring J, Rothenberg ME, Roufosse F, Sajous MH, Sheikh J, Simon D, Simon HU, Stein ML, Wardlaw A, Weller PF, Klion AD. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009; 124:1319–1325.e3.

3. Ogbogu PU, Rosing DR, Horne MK 3rd. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007; 27:457–475.

5. Parrillo JE, Borer JS, Henry WL, Wolff SM, Fauci AS. The cardiovascular manifestations of the hypereosinophilic syndrome. Prospective study of 26 patients, with review of the literature. Am J Med. 1979; 67:572–582.
6. Sen T, Gungor O, Akpinar I, Cetin M, Tufekcioglu O, Golbasi Z. Cardiac involvement in hypereosinophilic syndrome. Tex Heart Inst J. 2009; 36:628–629.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download