Abstract
Cardiac papillary fibroelastomas (CPF) are benign cardiac tumors and usually discovered incidentally during echocardiography. This report describes the case of a 68-year-old man, referred to cardiology for multiple masses of the left ventricle and left atrium. The transthoracic echocardiography revealed multiple oscillating masses in the left ventricle and aortic valve, non-mobile mass in the left atrium with severe mitral stenosis and moderate aortic regurgitation. The patient underwent surgical resection of the masses with valve replacements. Histopathologic examination confirmed the diagnosis of CPF in the left ventricle and aortic valve, thrombus in the left atrium.
After myxomas and lipomas, cardiac papillary fibroelastomas (CPF) are the most common benign primary tumors of the heart (7.9%).1) Pathologically, these tumors grossly appear like a "sea anemone", because they consist of multiple frond-like projections. Histologically, they are avascular tumors derived from normal components of the endocardium.2) CPF are usually discovered incidentally during a routine transthoracic echocardiography (TTE). Clinical neurologic, embolic and coronary ischemic symptoms have been reported in association with CPF. Surgical excision of the tumor is recommended for all patients who develop symptoms, but the treatment of asymptomatic patients with and echocardiographically identified CPF is still controversial.3)4) We present a case of multiple CPF in left ventricle and aortic valve with the left atrial thrombus, coincidentally discovered on echocardiography, and removed by a surgical resection.
A 68-year-old male with hypertension and atrial fibrillation, was admitted with progressive worsening dyspnea and chest pain. Physical examination at the time of the first admission revealed a temperature of 36.5℃; respiration 20/min; pulse, 100/min and irregular; and blood pressure, 120/80 mmHg. There was a prolonged diastolic murmur at the apex and laboratory data were unremarkable. Electrocardiogram revealed atrial fibrillation with rapid ventricular response. A chest X-ray showed cardiomegaly. TTE revealed a large left atrium of 7.22 cm diameter, severe mitral stenosis, mild mitral regurgitation, moderate aortic regurgitation, and presence of a multiple oscillating variable sized masses in the left ventricle and aortic valve, non-mobile 3.5 × 4.4 cm sized mass in the left atrium. The mitral valve leaflets were heavily thickened and calcified. The masses in the left ventricle were 0.31 × 0.92 cm, and 0.54 × 0.98 cm in size, oscillating heterogeneous echogenic material attached to the interventricular septum basal to mid level and 1.46 × 1.64 cm, 0.47 × 1.07 cm in size, mobile oval shaped mass, which had some echolucent area attached to the posterolateral papillary muscle and aortic valve (Fig. 1). The mass of the left atrium was 3.5 × 4.4 cm in size, non-mobile echogenic mass in the left atrium posterior wall. Cardiac magnetic resonance imaging demonstrated non-enhanced masses in the left atrium between the orifice of the right superior and inferior pulmonary vein and ventricle, which were heterogeneous in its signal intensity in T2 image (Fig. 2). Coronary angiography was normal. Based upon the findings as above, a differential diagnosis was made, which included thrombus, myxoma, fibroelastoma and inflammatory mass. In view of the possibility of embolism, unknown nature of the pathology and multiple valve diseases with symptoms, the patient was taken for urgent surgical resection with valve replacements.
Histopathology examination of the resected masses in the left ventricle and aortic valve revealed a papillary proliferation, including an avascular connective tissue core lined by a single layer of the endothelial cells, which was sufficient for a diagnosis of CPF (Fig. 3). The left atrial mass was composed of fibrin and red cells with a variable platelet and leukocyte component, revealed to thrombus.
The postoperative course was uncomplicated and the patient was discharged in a satisfactory condition on the 12th day.
CPF predominate in adults and are particularly frequent between the 4th and 8th decades of life. Most cases are probably acquired, however the etiology is unknown.5) CPF are more frequently located on the aortic valve (40%), tricuspid valve (17%), mitral valve (14%), pulmonary valve (13%), left atrium (7%), right atrium (2%), right ventricle (2%), and left ventricle papillary muscle (1%).6) Left ventricular CPF is rare, only reported via case reports.7) Although it is found incidentally, it can result in life-threatening complications, such as coronary and cerebral embolism, acute valvular dysfunction and sudden death.8) The most common clinical presentations are stroke, syncope, mesenteric ischemia, pulmonary emboli and sudden death.5) The clinical presentation is determined by location, size, and mobility of the tumor and when they arise from the left sided heart, systemic embolism is frequent. The treatment of choice of CPF is surgical excision, which is safe without causing significant morbidity or mortality. When valvular involvement is present, excision with valve repair or replacement is curative. Asymptomatic non-mobile or right side CPF could be followed-up closely.4)5)
The diagnostic method of choice for CPF is TTE or transesophageal echocardiography (TEE), although the ultimate diagnosis of CPF is based on histopathology. The most characteristic echocardiographic features that identify a tumor as a CPF are small size (usually < 1.5 cm), pedical or stalk attachment to endocardium, with high mobility, and refractive appearance and areas of echolucency within the tumor.2) Although no extensive studies have yet quantified the diagnostic yield of TEE for CPF compared with TTE, TEE is considered to be more accurate in diagnosing CPF. For tumors with a diameter < 0.2 cm, the sensitivity of TTE was only 61.9% and of TEE was 76.6%. In contrast, the sensitivity and specificity of TTE for CPF with a diameter > 0.2 cm are 88.9% and 87.8%, respectively.9) However, it is impossible to differentiate CPF from myxomas or thrombi, using TTE or TEE alone. Magnetic resonance imaging (MRI) may be more helpful than TEE in detecting the extent of the lesion invading the myocardium. MRI typically demonstrates a CPF mass on a valve leaflet or on the endocardial surface of the affected cardiac chamber and increase accuracy of diagnosis by showing the differential enhacement with respect to the surrounding normal cardiac structures.10)
Histopathologically, CPF are composed of a central stalk with radiating villus-like projections. The papillae are avascular structures, which contains a core of dense collagen fibers admixed with varying amounts of reticulin and elastin fibers. The cells lining the elongated papillae are hyperplastic endothelial cells, occasionally bulging from the surface.11) The lining epithelium is contiguous with the rest of endocardium.
This case demonstrates a surgical management of CPF and thrombus, diagnosed by echocardiography and cardiac MRI. This case of CPF is unusual with respect to the site of origin showing multiple involvement including the papillary muscle (its prevalence in the literature is only about 1% in CPF) and the large amount of thrombus in the left atrium.
Figures and Tables
Fig. 1
Small oval shaped masses seen in parasternal long axis view (A, arrows), parasternal short axis view (B, arrows), showing heterogeneous echogeneicity with some internal echolucency.
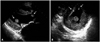
Fig. 2
Cardiac magnetic resonance imaging demonstrated non-enhanced masses in left atrium (A, arrow) between the orifice of the right superior and inferior pulmonary vein, and in left ventricle (B, arrow) showing heterogeneous signal intensity in T2 image.
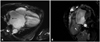
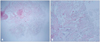
Fig. 3
Gross specimen of 1:1 paraffin block (H&E stain, × 10) (A) reveals central stalk with papillary projection. Histological examination of the resected tumor showing papillary projection. The tumor surface is covered by a single layer of endocardial cells with an overlying thin layer of an underlying acellular stroma composed of elastic fibers and collagen (H&E stain, × 40) (B).
References
1. Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R. Primary cardiac valve tumors. Ann Thorac Surg. 1991; 52:1127–1131.

2. Klarich KW, Enriquez-Sarano M, Gura GM, Edwards WD, Tajik AJ, Seward JB. Papillary fibroelastoma: echocardiographic characteristics for diagnosis and pathologic correlation. J Am Coll Cardiol. 1997; 30:784–790.

3. Mutlu H, Demir IE, Leppo J, Levy WK. Nonsurgical management of a left ventricular pedunculated papillary fibroelastoma: a case report. J Am Soc Echocardiogr. 2008; 21:877.e4–877.e7.

4. Seol SH, Kim DS, Han YC, Kim KH, Kim YB, Kim DK, Ung-Kim , Yang TH, Kim DK, Kim DI. Nonsurgical management of a tricuspid valvular pedunculated papillary fibroelastoma. Cardiovasc Ultrasound. 2009; 7:44.

5. Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003; 146:404–410.

6. Samoun M, Sansone F, Burlo M, Calafiore AM. Papillary fibroelastoma of the anterolateral papillary muscle: an unusual case. J Cardiovasc Med (Hagerstown). 2006; 7:830–832.

7. Park JH, Seol SH, Cho HJ, Park SH, Kim DK, Kim U, Yang TH, Kim DK, Kim DI, Kim DS. Papillary fibroelastoma presenting as a left ventricular mass. J Cardiovasc Ultrasound. 2010; 18:66–69.

9. Saloura V, Grivas PD, Sarwar AB, Gorodin P, Ledley GS. Papillary fibroelastomas: innocent bystanders or ignored culprits? Postgrad Med. 2009; 121:131–138.

10. Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics. 2000; 20:1073–1103. quiz 1110-1, 1112.

11. Fishbein MC, Ferrans VJ, Roberts WC. Endocardial papillary elastofibromas. Histologic, histochemical, and electron microscopical findings. Arch Pathol. 1975; 99:335–341.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download