Abstract
To date, it has been well documented that there is a relationship between alterations in thyroid hormones and cardiac dysfunction. We experienced a case of a 36-year-old man with dilated cardiomyopathy (DCM) accompanied by undiagnosed primary hypothyroidism. In the current case, there was a significant improvement in the cardiac function following heart failure management and thyroid hormone replacement. Our case highlights that clinicians should consider the possibility of hypothyroidism as a cause of DCM.
A reversible form of dilated cardiomyopathy (DCM) can be developed from alcohol drinking, pregnancy, chronic uncontrolled tachycardia, hypothyroidism, hyperthyroidism, drug use and other endocrine dysfunctions.1)2) Thyroid hormone has a great effect on the heart and vascular system.1) The heart is sensitive to changes in thyroid hormones, and cardiac disorders are commonly associated with both hyper- and hypothyroidism.3)4) Hemodynamic changes caused by hyperthyroidism lead to classic hyperdymamic cardiovascular state, and they are associated with increase in cardiac output and reduction in peripheral vascular resistance.5) On the other hand, hypothyroidism is associated with bradycardia, mild diastolic hypertension, narrow pulse pressure and slightly increased mean arterial pressure.6) According to a review of literatures, diastolic dysfunction is the most common finding seen in patients with hypothyroidism.7) In addition, it is commonly encountered that the left ventricular systolic function is minimally decreased with slightly reduced ejection fraction and stroke volume.8) DCM is a rare presentation of hypothyroidism.9)
We experienced a case of a 36-year-old man with DCM accompanied by undiagnosed primary hypothyroidism. Here, we report our case with a review of literatures.
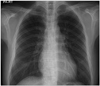
A 36-year-old man presented to the emergency room with dyspnea of New York Heart Association functional class III/IV and fatigue. The patient had a 1-year-history of chief complaints of weakness of all four extremities, weight gain and bilateral lower extremity edema. For two months prior to admission, the patient had a progressive worsening of bilateral lower extremity edema. On physical examination, the patient had body mass index (BMI) 28.6 kg/m2 and vital signs such as blood pressure 130/90 mmHg, pulse rate 90 beats/min, respiratory rate 20 breaths/min and O2 saturation 96% in room air. In addition, the patient had pale and dry skin. Heart rate was regular and systolic murmur was heard at the apex. Breath sounds were decreased with inspiratory crackles on bilateral lung bases. The patient also had bilateral presence of non-pitting edema of the foot and ankle. On chest X-ray, the patient had cardiomegaly with perihilar congestion and blunting of both costophrenic angles. These findings are suggestive of pleural effusion (Fig. 1). On electrocardiographic findings, the patient had normal sinus rhythm with low voltage of limb leads, interventricular conduction delay and non-specific ST-segment and T-wave changes (Fig. 2). On clinical laboratory examinations, the patient showed elevated serum levels of N-terminal pro-brain natriuretic peptide (5026 pg/mL) and normal range of cardiac enzymes. Renal functions were normal with blood urea nitrogen of 14.8 mg/dL and creatinine of 1.2 mg/dL. Lipid panel showed total cholesterol 231 mg/dL, triglyceride 188 mg/dL, high-density lipoprotein cholesterol 34 mg/dL and low-density lipoprotein cholesterol 88 mg/dL. Liver function test showed elevated serum levels of transaminases with total bilirubin 1.34 mg/dL, aspartate transaminase 402 IU/L, alanine transaminase 340 IU/L and alkaline phosphatase 44 IU/L. On complete blood counts, the patient had white blood cell counts 9260/mm3 with 56% neutrophils, hemoglobin 10.8 g/dL and platelets 190000/mm3. Serum electrolytes showed [Na+] = 120 meq/L, suggesting hyponatremia, and [K+] = 4.3 meq/L. Serum creatinine kinase was elevated (2738 U/L). On echocardiography, the patient had a dilated left ventricular cavity with a diastolic dimension of 6.1 cm, a decreased global systolic function with an ejection fraction of 16% and functional mitral regurgitation of moderate grade (Fig. 3). The patient had a ratio of transmitral early peak velocity (E) to septal mitral annulus velocity (E') of 13. The patient was started on loop diuretic therapy using furosemide and angiotensin converting enzyme (ACE) inhibitor (enalapril) for heart failure. On day 2, the patient underwent thyroid function test. This showed that the patient had elevated serum levels of thyroid stimulating hormone (100 µIU/mL) (reference range: 0.5-5 µIU/mL), decreased serum levels of T3 (60 ng/dL) (reference range: 80-180 ng/dL) and decreased serum levels of free T4 (0.054 ng/dL) (reference range: 0.7-1.9 ng/dL). Furthermore, the patient underwent additional tests to reveal the cause of hypothyroidism. This showed that the patient was positive for thyroglobulin antibody but negative for anti-microsomal one. On thyroid ultrasonography, the patient had an atrophic thyroid gland with hypoechoic parenchyma with two small nodules of 5 mm and 8 mm in size in the right lobe. On Tc 99-mm radionuclide thyroid scan, there was an increased uptake in the above two small nodules. On fine-needle aspiration biopsy of the nodules, the patient had adenomatous hyperplasia on lymphocytic thyroiditis background (Fig. 4). These findings were suggestive of Hashimoto's thyroiditis accompanied by atrophic autoimmune thyroiditis. Based on these findings, the patient was started on thyroid hormone replacement with thyroxine. Thyroxine dose was titrated up to 50 µg/day after two weeks and then up to 100 µg/day. This was followed by the adjustment of thyroxine dose based on thyroid functions. At a 1-year follow-up, the patient had a gradual decrease in the enlarged left ventricular chamber and a normalization of the decreased left ventricular systolic functions (Fig. 5). Furthermore, the patient also had a normalization of clinical laboratory findings such as transaminases and creatinine kinase. Follow-up echocardiography findings are shown in Table 1. Three months after the treatment, the patient had a decrease in the BMI to 23.4 kg/m2. The patient discontinued use of loop diuretics (furosemide) but continued use of ACE inhibitor (enalapril), β-blockers (bisoprolol) and thyroid hormone replacement therapy.
Hypothyroidism is associated with decreased cardiac contractility, increased systemic vascular resistance and decreased cardiac output. Its manifestations are insidious and subtle in its progression and clinical behavior.4) DCM, the most common form of cardiomyopathy, may be caused by various factors such as metabolic/endocrine disturbances, electrolyte imbalances, inflammation, infection, immune system disorders and toxins.10) Thyroid hormones act on the cardiac myocyte and peripheral vasculature. The genomic and non-genomic effects of thyroid hormone are related to the cardiac function and cadiovascular hemodynamics.4) To explain their possible genomic effects on the cardiovascular system, it has been proposed that they involved in the regulation of the mRNA transcription of genes associated with the contractile system.1) They have a non-genomic effects on the ionic channels of cardiomyocyte's membrane.4) Patients with hypothyroidism present with cardiovascular manifestations such as bradycardia, decreased contractility of the myocardium, increased systemic peripheral vascular resistance and the pericardial effusion. Moreover, patients with hypothyroidism are at increased risks of developing atherosclerosis and ischemic heart disease.11)12) It has been reported that the cardiac manifestations are associated with alterations in the expression of T3-mediated genes in patients with thyroid dysfunction.4) Patients with hypothyroidism are less likely to develop heart failure although their cardiac output is decreased. This is due to their lower degree of oxygen demand.13)
In the current case, the patient had no family history of DCM. In addition, the patient also had no past history of alcohol abuse or history of other drug or substance addiction. The echocardiographic findings were suggestive of DCM. The patient was well responsive to hormone replacement therapy. The patient achieved euthyroid status and had a normalization of the left ventricular ejection fraction and left ventricular size. This indicates that there is a possible relationship between the hypothyroidism and the development of the left ventricular dysfunction in the current case. Moreover, our case indicates that myocardial function in DCM secondary to hypothyroidism can be reversed with restoration of normal thyroid function and the management of heart failure.
In conclusion, our case highlights that clinicians should consider the possibility of DCM secondary to hypothyroidism in patients with congestive heart failure.
Figures and Tables
Fig. 1
Initial chest X-ray. A posteroanterior chest X-ray view shows cardiomegaly and both pleural effusions before treatment.
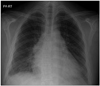
Fig. 2
The 12-lead electrocardiography findings. On admission, the patient had normal sinus rhythm with low voltage of limb leads, interventricular conduction delay and nonspecific ST-segment and T-wave changes.
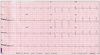
Fig. 3
Color Doppler of mitral regurgitation. A: Initial color Doppler findings. B: At a follow-up, there was no mitral regurgitation.
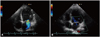
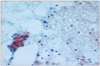
Fig. 4
A fine-needle aspiration biopsy findings. A fine-needle aspiration biopsy of the nodule shows adenomatous hyperplasia in a background of lymphocytic thyroiditis (Papanicolaou's stain, × 450).
References
1. Khochtali I, Hamza N, Harzallah O, Hamdi S, Saad J, Golli M, Mahjoub S. Reversible dilated cardiomyopathy caused by hypothyroidism. Int Arch Med. 2011; 4:20.

2. Sung JK, Kim JY, Ryu DW, Lee JW, Youn YJ, Yoo BS, Choe KH. A case of hypocalcemia-induced dilated cardiomyopathy. J Cardiovasc Ultrasound. 2010; 18:25–27.

3. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001; 344:501–509.

5. Fazio S, Palmieri EA, Lombardi G, Biondi B. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res. 2004; 59:31–50.

6. McAllister RM, Delp MD, Laughlin MH. Thyroid status and exercise tolerance. Cardiovascular and metabolic considerations. Sports Med. 1995; 20:189–198.
7. Wieshammer S, Keck FS, Waitzinger J, Henze E, Loos U, Hombach V, Pfeiffer EF. Acute hypothyroidism slows the rate of left ventricular diastolic relaxation. Can J Physiol Pharmacol. 1989; 67:1007–1010.

8. Crowley WF Jr, Ridgway EC, Bough EW, Francis GS, Daniels GH, Kourides IA, Myers GS, Maloof F. Noninvasive evaluation of cardiac function in hypothyroidism. Response to gradual thyroxine replacement. N Engl J Med. 1977; 296:1–6.

9. Kumar Kota S, Tripathy PR, Krishna Kota S, Jammula S, Kumar Meher L, Modi KD. Primary hypothyroidism: uncommon presentation with reversible dilated cardiomyopathy in a young subject. Int J Endocrinol Metab. 2012; 10:440–443.

10. Kuethe F, Sigusch HH, Bornstein SR, Hilbig K, Kamvissi V, Figulla HR. Apoptosis in patients with dilated cardiomyopathy and diabetes: a feature of diabetic cardiomyopathy. Horm Metab Res. 2007; 39:672–676.

12. François M, Delemer B. {What’s new in the couple thyroid and heart in 2008?}. Ann Endocrinol (Paris). 2008; 69:Suppl 1. S37–S43.
13. Stănescu C, Branidou K, Ranetti EA. Heart failure and dilated cardiomyopathy associated with severe longstanding untreated hypothyroidism. Rom J Intern Med. 2007; 45:77–83.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download