Abstract
Left atrial dissection (LatD), defined as the forced separation of the left atrial (LA) wall layers by blood, is a rare and severe complication of cardiac surgery. It is most frequently associated with atrioventricular junction injuries. We report a case of infected LatD after coronary artery bypass graft, mitral valve replacement, aortic valve replacement and ascending aortic root replacement. The patient was presented with septicemia and disseminated intravascular coagulation. To the best of our knowledge, this is the first case report of LA dissecting flap concomitant with attached infective vegetations identified by transesophageal echocardiography.
Forced separation of the left atrial (LA) wall layers by blood, is known as left atrial dissection (LatD). LatD is a rare and severe complication of cardiac surgery1) occurring in up to 0.84% of mitral valve replacements (MVR).2)
Other rarer causes include mitral valve (MV) repair, aortic valve (AV) surgeries, myocardial infarction, percutaneous coronary intervention, cardiac mass excision, left ventricular (LV) aneurysm repair, coronary artery bypass graft (CABG), blunt cardiac trauma, pulmonary vein cannulation, infective endocarditis (IE) and spontaneous occurrence.1)
It is most frequently associated with atrioventricular junction injuries. However, there are other less common cases including those remote from the atrioventricular junction.1)
We report a case of infected LatD after CABG, MVR, AV and ascending aortic root replacement which was presented with disseminated intravascular coagulation (DIC) and septicemia.
To the best of our knowledge, this is the first report of LA dissecting flap associated with infective vegetations which was identified by transesophageal echocardiography (TEE).
A 69-year-old man was admitted in our hospital due to fever, chill, orthopnea and paroxysmal nocturnal dyspnea. In physical examination, respiratory sounds were reduced in both his lower half of lung fields.
He had undergone a major cardiac surgery including CABG, MVR, aortic valve replacement (AVR) and ascending aorta replacement due to severe coronary artery disease, significant valvular heart disease (rheumatic) and aneurysmally dilated ascending aorta, 75 days prior to his recent admission.
An electrocardiogram revealed atrial fibrillation rhythm, normal axis and non-specific ST-T changes in inferior and lateral leads (Fig. 1). The posteroanterior view of the chest radiography showed massive bilateral pleural effusion. Transthoracic echocardiography followed by TEE showed normal LV size with preserved systolic function, bioprosthetic MV with acceptable hemodynamic and mobility and no paravalvular regurgitation. Bioprosthetic AV had acceptable hemodynamic and mobility without paravalvular regurgitation. There was normal functioning ascending aorta Dacron tube graft without leakage from proximal and distal part of prosthesis.
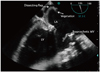
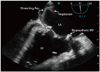
The LA was enlarged and dissection was found on the posterior wall. Two homogenous particles (1.18 × 0.4 cm and 0.9 × 0.58 cm) were attached to the dissecting flaps which were highly suggestive for vegetations and IE (Fig. 2, 3, and 4, Supplementary movie 1, 2, and 3).
Laboratory tests showed severe coagulopathy, high levels of fibrin degradation products, leukocytosis (white blood cells count: 26700/cm3, predominantly poly morphonucleated), severe anemia, thrombocytopenia, elevated liver enzymes and serum creatinine levels. These data were suggestive for DIC. The blood culture revealed enterococci septicemia.
His general condition deteriorated rapidly. He suffered from tonic-clonic seizures, diminished consciousness, widespread mucosal bleeding and oliguria. Bilateral chest tubes were inserted for massive pleural effusion. The patient was treated with intravenous vancomycin, cefepim, gentamycin and oral rifampin. He received infusion of fresh frozen plasma, packed cells, valproic acid, diazepam and phenytoin.
Based on his poor general condition, cardiac surgery was not performed and finally he expired from multi organ failure. Autopsy findings were consistent with clinical, laboratory and echocardiographic findings.
LatD is defined as a gap from the mitral annular area to the LA wall, developing a new cavity with or without connection into the true LA. The false chamber presents as an echolucent area and may cause partial elimination of atrial cavity.3) Similar to what happens in aortic dissection, the false chamber may be compressed during ventricular systole as LA is being filled.4)
The etiology of LatD after MVR is unknown. Suggested causes include: inappropriate suturing of the annulus to the prosthesis, inadvertent trauma to the posterior part of mitral annulus, intense debridement of the annulus, intense tension on posterior annulus sutures and inadvertent injury to the LA endocardium during atrial thrombectomy.5)
Suggested etiologies for LatD after AVR are division of the non-coronary aortic annulus and entrance into the LA wall due to removal of calcification or inadvertent incision of LV outflow tract.2)
LA wall is formed by two muscular layers that are longitudinally and circumferentially aligned. Disruption in this site may be a predisposing factor for LatD.2)
In our case, dissecting flap was remote from the atrioventricular junction. Therefore, flap was probably induced by unintended LA endocardial injury. Time to presentation of LatD after MVR ranged from 1 day to 9 years.3) In our patient, it was diagnosed after seventy five days.
Reported clinical presentations include: acute low output syndrome and congestive heart failure in the immediate post-operative period, symptoms of moderate cardiac failure, persistent fever or it may be an incidental finding during routine echocardiography assessment.3)
Our patient presented as persistent fever and progressive dyspnea. Dyspnea might be caused by bilateral severe pneumonia with parapneumonic effusion, septicemia and DIC. TEE clearly improved the diagnostic capacity of echocardiography as a technique of choice in the semi-invasive diagnosis of LatD.3) LatD usually requires surgical treatment to stop blood flow to the false chamber.3)
Rupture of atrial wall with fistula formation into the LA or rupture into the pericardial space with bleeding or tamponade may occur. Also, pulmonary vein obstruction with reduced blood return and pulmonary edema have been reported.3) In patients with LatD and no or minimal symptoms, close follow-up is recommended.5)
In conclusion, previous cardiac surgery seems to be the main risk factor for developing LatD. Our study showed that a complicated infective flap in LA wall can be a source for producing septic systemic emboli and DIC. To the best of our knowledge, this form of LA dissecting flap has not been reported in the literature previously.
Figures and Tables
 | Fig. 1An electrocardiogram revealed atrial fibrillation rhythm, normal axis and non-specific ST-T changes in inferior and lateral leads. |
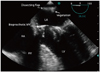 | Fig. 2Transesophageal echocardiography in mid esophageal four chamber view (0 degree) demonstrated a linear dissecting flap and its attached (irregular shaped) particles originated from left atrial wall. LA: left atrium, LV: left ventricle, MV: mitral valve, RA: right atrium, RV: right ventricle. |
References
1. Fukuhara S, Dimitrova KR, Geller CM, Hoffman DM, Ko W, Tranbaugh RF. Left atrial dissection: etiology and treatment. Ann Thorac Surg. 2013; 95:1557–1562.

2. Leissner KB, Srinivasa V, Beutler S, Matyal R, Badr R, Haime M, Mahmood FU. Left atrial dissection and intramural hematoma after aortic valve replacement. J Cardiothorac Vasc Anesth. 2011; 25:309–310.

3. Gallego P, Oliver JM, González A, Domínguez FJ, Sanchez-Recalde A, Mesa JM. Left atrial dissection: pathogenesis, clinical course, and transesophageal echocardiographic recognition. J Am Soc Echocardiogr. 2001; 14:813–820.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download