Abstract
Background
The non-invasive differentiation of ischemic and nonischemic acute heart failure (AHF) not resulting from acute myocardial infarction is difficult and has therapeutic and prognostic implications. The aim of this study was to assess whether resting myocardial contrast echocardiography (MCE) can detect coronary artery disease (CAD) in patients with decreased left ventricular (LV) systolic function and global hypokinesis presenting with AHF.
Methods
Twenty-one consecutive patients underwent low-power real-time MCE based on color-coded pulse inversion Doppler. Standard apical LV views were acquired during contrast IV infusion of Definity®. Following transient microbubbles destruction, the contrast replenishment rate (β), reflecting myocardial blood flow velocity, was derived by plotting signal intensity vs. time and fitting data to the exponential function: y (t) = A (1 - e-β(t-t0)) + C.
Results
Of the 21 (mean age 56.6 ± 13.6 years) patients, 5 (23.8%) demonstrated flow-limiting CAD (> 70% of luminal diameter narrowing). The mean ± standard deviation of LV ejection fraction was 29.6 ± 8.6%. Quantitative MCE analysis was feasible in 258 of 378 segments (68.3%). There were no significant difference in "β" and "Aβ" in patients without and with CAD (0.48 ± 0.27 vs. 0.45 ± 0.25, p = 0.453 for β and 2.99 ± 2.23 vs. 3.68 ± 3.13, p = 0.059 for Aβ, respectively). No contrast-related side effects were reported.
Myocardial ischemia is one of the most prevalent cause of acute heart failure (AHF).1)2) Ascertaining myocardial ischemia is essential to treatment and prognosis.3,4,5) Patients with ischemic etiology may benefit from revascularization and show different response to drug treatment.6)7) Furthermore, ischemic origin has a poorer prognosis and the mechanism of sudden death may differ between ischemic and nonischemic heart failure patients,8) further emphasizing the potential importance of accurate differential diagnosis between origins. Clinically, it often is difficult to distinguish patients with and without underlying coronary artery disease (CAD) as the cause of AHF in the absence of a prior history of CAD or typical electrocardiogram (ECG) changes and elevation of cardiac enzyme suggesting diagnosis of acute coronary syndrome (ACS). Regional wall motion abnormalities (RWMAs) on echocardiography are known to be useful to detect CAD, however, some patients show global hypokinesis rather than RWMA.9) Although noninvasive functional testing, i.e., stress radionuclide perfusion imaging, stress echocardiography testing, or cardiovascular magnetic resonance imaging has been recommended to detect CAD,10) these tests cannot be done on bedside nor immediately after admission. Myocardial contrast echocardiography (MCE) uses intravenously administered contrast agent that traverses the microvasculature and several studies have shown accuracy of MCE in detecting flow-limiting CAD in patients with suspected CAD during vasodilator stress.11,12,13) However, MCE with vasodilator stress is also not feasible in acute setting in patients with AHF. In one study, it took a mean of 9 ± 2 days after admission to perform MCE with dipyridamole stress in patients with AHF.14) Therefore, early implementation of medications that usually not recommended in AHF patients such as antiplatelet agents or statins could be delayed.
A study with the blood perfusion imaging obtained in the basal condition by positron emission tomography have shown reduced blood flow distribution in patients with ischemic cardiomyopathy compared with normal blood flow in patients with idiopathic cardiomyopathy.15) We hypothesized that resting MCE performed immediately after admission in patients with decreased LV ejection fraction (LVEF) may also be able to classify patients with myocardial ischemia. In this study, we sought to investigate the feasibility and usefulness of resting MCE for the early detection of flow-limiting CAD in patients presenting with first onset of AHF not caused by acute myocardial infarction (AMI).
Consecutive patients presenting to the hospital with newonset significant dyspnea and a clinical diagnosis of heart failure based on Framingham criteria and whose baseline echocardiography showed decreased LV systolic function (LVEF < 45%) without RWMA (i.e., global hypokinesis) were included in the study. Patients excluded from the study were those with typical ST elevation or ST depression on ECG with creatine kinase rise of more than twice the normal values, past history of documented AMI, history of significant valvular heart disease, myocardial revascularization, or ACSs. The study was approved by the Institutional Ethics Committee and conducted according to the Declaration of Helsinki.
A standard 12-lead ECG at the time of admission was recorded. All patients underwent transthoracic echocardiography in standard apical and parasternal views (M3S probe, Vivid 7 GE Healthcare, Horten, Norway) to assess RWMAs and global LVEF within 24 hours of admission. MCE was performed in patients who showed global hypokinesis at a mean 1 ± 1 days. For this pilot study, the data of 21 patients who underwent coronary arteriography before discharge from hospital were analyzed, although resting MCE was performed in 33 individuals during study period.
MCE was performed in the 3 apical views (i.e., apical 4-, 2-, and 3-chamber views) using low-power-continuous, power-modulation MCE at a mechanical index (MI) of 0.11. Background gains were set so than minimal tissue signal was seen. The color gains were then adjusted so that no Doppler signal was seen except at the mitral valve and proximal to apex. All patients underwent infusion of Definity (Lantheus Medical Imaging, Inc., North Billerica, MA, USA) at a rate of 240 mL/h, maintaining a homogenous contrast enhancement in the myocardium with no attenuation. Depletion-replenishment technique was used, in which a brief high-MI (1.2) "flash" impulse (duration 5-10 frames) was manually triggered, followed by low MI (MI = 0.11) imaging for up to 15 cardiac cycles. Nonstandard apical views were used, if required, to attempt to overcome basal attenuation artifacts. In large hearts, each myocardial wall (i.e., inferior and anterior walls) was imaged separately when artifacts were observed in the peripheral fields.
The myocardial segments were assigned to the coronary artery perfusion territories. A semiquantitative scoring system was used as follows: homogenous contrast perfusion = 2; partial/patchy contrast perfusion = 1; and absent contrast perfusion = 0.
Quantification of resting MCE images was performed off-line on a PC workstation. Analyses of the cineloops were performed blinded in random order using EchoPAC PC™ (GE Vingmed Ultrasound, Horten, Norway). Measurement of mean signal intensity (dB) was done in manually placed, equally sized and shaped regions of interest (ROI) in the 16 standard myocardial segments, plus the two apical segments of the apical long-axis view.16) When necessary, their position was slightly adjusted to compensate for translation of the heart. The depth of the ROI was not changed. The myocardial signal intensity (SI) was plotted against time (t) and fitted to the exponential function: y (t) = A (1 - e-β(t-t0)) + C, where y is SI at any time during contrast replenishment, "A, dB" is the plateau SI corresponding to the microvascular cross-sectional area or myocardial blood volume, "β, Sec-1" represented the rate of rise to the plateau reflecting myocardial blood velocity, "C" is the intercept at the origin reflecting the background intensity level. The introduction of t0 simply reflects that the analysis software allowed one to choose where to set t = 0. To further compensate for possible non-zero initial value after flash, the constant C was added, implicating that the curve fitting was relatively independent of background myocardial SI. Segmental values of A and β were derived from the replenishment cycles by careful frame-by-frame analysis. The product of A × β reflected an index of myocardial blood flow (MBF).17)
MCE images were analyzed separately by independent observers who were blinded to clinical, angiographic, and other respective imaging data. Qualitative and quantitative MCE data were also analyzed separately by two different observers.
Significant CAD was defined as the presence of > 70% luminal diameter narrowing of ≥ 1 major epicardial arteries or major branches.
Continuous variables were analyzed using Student's t-test, and dichotomous variables were analyzed using the chi square test. Data showed as mean ± standard deviations (SD) or number (%), and all variables that had a p value of 0.05 or less were considered statistically significant. We used statistical package SPSS version 19.0 (SPSS Inc., Chicago, IL, USA).
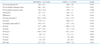
Table 1 describes the study population. Of the 21 patients (mean age 56.6 ± 13.6 years, range 37-81), 5 (23.8%) demonstrated flow-limiting CAD (> 70% of luminal diameter narrowing). There were no significant differences in patient demography, coronary risk factors, and echocardiographic parameters including LVEF (Table 2) in patients with and without CAD. The mean ± SD of LVEF and LV internal dimensions (diastole/systole) were 29.6 ± 8.6% and 60.8 ± 9.0/51.1 ± 9.5 mm, respectively, and the mean ± SD B-type natriuretic peptide level at admission was 1897.9 ± 1319.1 pg/dL.
Ultrasound contrast agents were administered to all 21 patients. There were no immediate or short-term (≤ 24 h) adverse events from contrast infusion.
From qualitative MCE analysis, only one patient showed partial perfusion in basal inferior, mid-inferior, basal inferolateral and mid inferolateral wall whose coronary arteriography confirmed the total occlusion of right coronary artery (RCA) and left circumflex artery (LCx) with 70% stenosis in mid left anterior descending artery (LAD) (Fig. 1). The patient was an old lady (81 years old), and refused the coronary bypass graft surgery.
Quantitative MCE analysis was feasible in 258 of 378 segments (68.3%) [97 of 126 (77.0%) segments in the 4-chamber view, 81 of 126 segments (64.3%) in the 2-chamber view, and 80 of 126 segments (63.5%) in the 3-chamber view]. The most frequent dropouts were observed in the basal segments of the lateral and anterior wall. According to coronary artery perfusion territories, quantitative analysis was feasible in 143 of 231 (61.9%) segments in the LAD territory, 56 of 84 (66.7%) of LCx territory, and 59 of 63 (93.7%) segments of RCA territory.
MCE parameters of A, β, and Aβ are shown in Table 3. "A" value was significantly higher in patient with CAD (p = 0.010) and there were no significant difference in "β" and "Aβ" in patients without and with CAD (0.48 ± 0.27 vs. 0.45 ± 0.25, p = 0.453 for β and 2.99 ± 2.23 vs. 3.68 ± 3.13, p = 0.059 for Aβ, respectively).
We hypothesized that resting MCE may help to classify patients with flow-limiting CAD presenting with an unknown cause of AHF with decreased LVEF. Resting quantitative MCE analysis was feasible, however, neither qualitative nor quantitative data of MCE helped to differentiate patients with CAD from patients without CAD.
In our study, a quarter of patients presenting AHF showed significant coronary artery stenosis (> 70%). This is relatively small numbers, for in OPTIMIZE-HF study, 66% of patients with LV systolic dysfunction carried a diagnosis of CAD.7) We included only patients who had no known risk factors of indicating CAD (ischemic symptom, electrocardiographic change, rise of cardiac enzyme and RWMA in baseline echocardiography), because detection of CAD is most challenging in such patients. We found that many patients showed RWMA in baseline echocardiography or had elevated cardiac enzyme on admission, so only small number of patients matched the inclusion criteria of the study. However, one may be able to assume that if baseline echocardiography in patients with no clinical evidence indicating CAD showed decreased LVEF and global hypokinesis, CAD is accompanied with relatively low probability, based on this result.
Quantitative MCE analysis was feasible in 68.3% of LV segments. This low feasibility has been reported by other groups.16)18) If the study subjects were not screened for echocardiographic image quality, feasibility of quantitative analysis was around 70%, and this may reflect the real-world setting of performing MCE.
Resting MCE did not distinguish patients with CAD from patients without CAD. There could be two explanations for this negative result. One is that resting MBF velocity may be normal despite the presence of coronary artery stenosis because of coronary arteriolar vasodilation or collateral MBF from non-flow limiting coronary arteries.19)20) Vasodilator stress can help the detection of CAD based on the occurrence of reversible perfusion defects, because myocardial blood flow velocity increases with vasodilator and MBF velocity reserve decreases with coronary artery stenosis.21) Many studies including of which aim was to differentiate ischemic cardiomyopathy from nonischemic cardiomyopathy showed that perfusion was also decreased in nonischemic cardiomyopathy.14)22)23) Abdelmoneim et al.24) proposed wall motion abnormality in Takotsubo cardiomyopathy could be made by microvascular dysfunction as evidenced by abnormal myocardial perfusion detected in MCE. In our study, the cause of LV systolic dysfunction is not known yet, although hypertensive cardiomyopathy or idiopathic cardiomyopathy could be the most probable diagnosis. In acute stage of heart failure, myocardial perfusion became abnormal even without significant coronary artery stenosis, and that can be the other explanation of no differences of myocardial velocity (β) and MBF (Aβ) between patients with and without CAD in our study.
The present study had several limitations. First, the population was small and thus, these negative findings could be caused by a low statistical power. Because some reports showed RWMA is not sensitive for detection of CAD in patients with dilated LV, exclusion of patients with RWMA could make this study have less power. Second, only resting MCE was considered in our study. Some reports showed diagnostic value of stress MCE for the detection of CAD, however, in acute setting, it is impossible to perform stress test in patients with AHF. So, this study was designed to perform resting MCE only, immediately after admission.
In conclusion, relatively small number of patients had CAD in patients with decreased LV systolic function with global hypokinesis. Resting quantitative MCE analysis was feasible in patients presenting as AHF, however, qualitative or quantitative data of MCE did not aid to differentiate patients with CAD from patients without CAD.
Figures and Tables
Fig. 1
Case of 81-year-old female. Apical 4-chamber, 2-chamber, and 3-chamber view showed partial perfusion defect in inferior and inferolateral wall (arrows, A). Coronary arteriography showed 70% stenosis in mid left anterior descending artery, and total occlusion of right coronary artery and left circumflex artery (B).
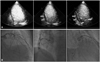
Table 1
Patients demographic data, heart rate, and BNP in patients without and with significant CAD (> 70% diameter stenosis)

Acknowledgements
This study was supported by a research grant from the Korean Society of Echocardiography.
References
1. Gheorghiade M, Sopko G, De Luca L, Velazquez EJ, Parker JD, Binkley PF, Sadowski Z, Golba KS, Prior DL, Rouleau JL, Bonow RO. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006; 114:1202–1213.

2. Fox KF, Cowie MR, Wood DA, Coats AJ, Gibbs JS, Underwood SR, Turner RM, Poole-Wilson PA, Davies SW, Sutton GC. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J. 2001; 22:228–236.

3. Bart BA, Shaw LK, McCants CB Jr, Fortin DF, Lee KL, Califf RM, O'Connor CM. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. J Am Coll Cardiol. 1997; 30:1002–1008.

4. Purek L, Laule-Kilian K, Christ A, Klima T, Pfisterer ME, Perruchoud AP, Mueller C. Coronary artery disease and outcome in acute congestive heart failure. Heart. 2006; 92:598–602.

5. Flaherty JD, Rossi JS, Fonarow GC, Nunez E, Stough WG, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, Yancy CW, Young JB, Davidson CJ, Gheorghiade M. Influence of coronary angiography on the utilization of therapies in patients with acute heart failure syndromes: findings from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J. 2009; 157:1018–1025.

6. Follath F, Cleland JG, Klein W, Murphy R. Etiology and response to drug treatment in heart failure. J Am Coll Cardiol. 1998; 32:1167–1172.

7. Rossi JS, Flaherty JD, Fonarow GC, Nunez E, Gattis Stough W, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, Yancy CW, Young JB, Davidson CJ, Gheorghiade M. Influence of coronary artery disease and coronary revascularization status on outcomes in patients with acute heart failure syndromes: a report from OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure). Eur J Heart Fail. 2008; 10:1215–1223.

8. Uretsky BF, Thygesen K, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Packer M, Poole-Wilson PA, Ryden L. Acute coronary findings at autopsy in heart failure patients with sudden death: results from the assessment of treatment with lisinopril and survival (ATLAS) trial. Circulation. 2000; 102:611–616.

9. Medina R, Panidis IP, Morganroth J, Kotler MN, Mintz GS. The value of echocardiographic regional wall motion abnormalities in detecting coronary artery disease in patients with or without a dilated left ventricle. Am Heart J. 1985; 109:799–803.

10. Chrysohoou C, Greenberg M, Stefanadis C. Non-invasive methods in differentiating ischaemic from non-ischaemic cardiomyopathy. A review paper. Acta Cardiol. 2006; 61:454–462.

11. Jeetley P, Hickman M, Kamp O, Lang RM, Thomas JD, Vannan MA, Vanoverschelde JL, van der Wouw PA, Senior R. Myocardial contrast echocardiography for the detection of coronary artery stenosis: a prospective multicenter study in comparison with single-photon emission computed tomography. J Am Coll Cardiol. 2006; 47:141–145.

12. Firschke C, Andrássy P, Linka AZ, Busch R, Martinoff S. Adenosine myocardial contrast echo in intermediate severity coronary stenoses: a prospective two-center study. Int J Cardiovasc Imaging. 2007; 23:311–321.

13. Senior R, Moreo A, Gaibazzi N, Agati L, Tiemann K, Shivalkar B, von Bardeleben S, Galiuto L, Lardoux H, Trocino G, Carrió I, Le Guludec D, Sambuceti G, Becher H, Colonna P, Ten Cate F, Bramucci E, Cohen A, Bezante G, Aggeli C, Kasprzak JD. Comparison of sulfur hexafluoride microbubble (SonoVue)-enhanced myocardial contrast echocardiography with gated single-photon emission computed tomography for detection of significant coronary artery disease: a large European multicenter study. J Am Coll Cardiol. 2013; 62:1353–1361.

14. Senior R, Janardhanan R, Jeetley P, Burden L. Myocardial contrast echocardiography for distinguishing ischemic from nonischemic first-onset acute heart failure: insights into the mechanism of acute heart failure. Circulation. 2005; 112:1587–1593.

15. Boff GM, Zanco P, Della Valentina P, Cardaioli P, Thiene G, Chioin R, Dalla Volta S. Positron emission tomography is a useful tool in differentiating idiopathic from ischemic dilated cardiomyopathy. Int J Cardiol. 2000; 74:67–74. discussion 75-6.

16. Malm S, Frigstad S, Helland F, Oye K, Slordahl S, Skjarpe T. Quantification of resting myocardial blood flow velocity in normal humans using real-time contrast echocardiography. A feasibility study. Cardiovasc Ultrasound. 2005; 3:16.

17. Wei K. Approaches to the detection of coronary artery disease using myocardial contrast echocardiography. Am J Cardiol. 2002; 90:48J–58J.

18. Abdelmoneim SS, Martinez MW, Mankad SV, Bernier M, Dhoble A, Pellikka PA, Chandrasekaran K, Oh JK, Mulvagh SL. Resting qualitative and quantitative myocardial contrast echocardiography to predict cardiac events in patients with acute myocardial infarction and percutaneous revascularization. Heart Vessels. 2014; [Epub ahead of print].

19. Jayaweera AR, Wei K, Coggins M, Bin JP, Goodman C, Kaul S. Role of capillaries in determining CBF reserve: new insights using myocardial contrast echocardiography. Am J Physiol. 1999; 277(6 Pt 2):H2363–H2372.
20. Janardhanan R, Burden L, Senior R. Usefulness of myocardial contrast echocardiography in predicting collateral blood flow in the presence of a persistently occluded acute myocardial infarction-related coronary artery. Am J Cardiol. 2004; 93:1207–1211.

21. Kaul S. Myocardial contrast echocardiography: a 25-year retrospective. Circulation. 2008; 118:291–308.
22. Watzinger N, Lund GK, Saeed M, Reddy GP, Araoz PA, Yang M, Schwartz AB, Bedigian M, Higgins CB. Myocardial blood flow in patients with dilated cardiomyopathy: quantitative assessment with velocity-encoded cine magnetic resonance imaging of the coronary sinus. J Magn Reson Imaging. 2005; 21:347–353.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download