Abstract
Cardiac calcified amorphous tumors (CATs) can arise in all four chambers of the heart. Cardiac CATs can cause diverse symptoms according to their locations, and mass or embolic effects. Pulmonary emboli arising from cardiac CATs have been reported, but the true incidence is unknown due to their rarity. Herein we report a rare case with diffuse CATs in the right ventricle which caused a calcific pulmonary embolism and right-sided heart failure. Echocardiography, chest non-contrast computed tomography, and cardiac magnetic resonance imaging helped us diagnose the CATs. We recommend the usefulness of a multimodality imaging approach to characterize intracardiac masses and their complications accurately.
Cardiac calcified amorphous tumors (CATs) are extremely rare cardiac masses which can arise in all four cardiac chambers.1)2) While several causes of cardiac CATs have been suggested, the true etiology is not still clear. Cardiac CATs are usually benign, but sometimes cause diverse symptoms due to obstruction or embolization.1)3)
We recently encountered a patient with a cardiac CAT causing multiple, calcific, pulmonary emboli and right-sided heart failure. A cardiac CAT has not been reported previously in Korea.
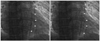
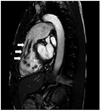
A 33-year-old man sought evaluation in our outpatient clinic for progressive pretibial pitting edema and shortness of breath on exertion. There was no personal or family history of thromboembolic, hematologic, inflammatory, or malignant diseases. He had no history of chest trauma. The physical examination revealed mild icteric sclera, a palpable liver, a distended abdomen, and jugular vein distention. A grade II pansystolic murmur was noted on the left parasternal border. A chest X-ray showed a dense calcification within the cardiac silhouette from the left lateral view. An electrocardiogram revealed incomplete right bundle branch block, right atrial enlargement, and right ventricular hypertrophy. Echocardiography demonstrated a diffuse calcified mass affecting the tricuspid chordal apparatus and the free wall of the right ventricle (RV), resulting in severe tricuspid regurgitation and markedly increased RV systolic pressure (70 mmHg) (Fig. 1). On laboratory analysis, the serum parathyroid hormone of 50.07 pg/mL (normal, 15-65 pg/mL), calcium of 8.1 mg/dL (normal, 8.0-10.5 mg/dL), creatinine of 1.0 mg/dL (normal, 0.5-1.4 mg/dL), and glucose of 100 mg/dL (normal, 70-110 mg/dL) levels were within normal limits. Hypereosinophilia was not noted. A chest computed tomography (CT) showed multiple pulmonary thromboemboli, possibly calcific, which were noted on non-contrast CT imaging (Fig. 2). Fluoroscopic imaging showed an irregular-shaped calcified mass in the RV which changed in shape and size during the cardiac cycle (Fig. 3). Cardiac magnetic resonance imaging (MRI) demonstrated a tubular calcified mass, which was separated from the right ventricular myocardium, extending from just below the tricuspid valve annulus to the right ventricular outflow tract, suggesting a CAT or calcific fibroma (Fig. 4). An endomyocardial biopsy was not performed due to the risk of right ventricular rupture or prolapse. Heart-lung transplantation was deferred until the pulmonary arterial pressure improved and empirical anticoagulation was administered.
A cardiac CAT was first reported in 1997.4) Cardiac CATs can arise in all four chambers of the heart,1)2) although the proportion in each chamber is not known.
Although mural thrombi,4)5) chest trauma,1) and increased parathyroid hormone and calcium phosphate product levels in hemodialysis patients6) have been described as the causes for cardiac CATs, the pathogenesis of these lesions is still unknown. In the present case, there was no identifiable cause.
Histologically, a cardiac CAT is characterized by nodular calcium deposits over a matrix of fibrin and/or amorphous fibrin-like material, hyalinization, inflammatory cells, and degenerated hematologic elements.7) Clinical tests usually show cardiac CATs to be benign, although they may cause obstruction or embolism,4) and cases can evolve fatally.5) An endomyocardial biopsy was not performed because of the risk of right ventricular rupture and further calcific embolization.8)
Surgical removal of the tumor may be indicated if embolism has occurred or seems likely. Complete surgical resection should be pursued if possible, considering its recurrence.5) Heart transplantation may be considered if not possible. We chose heart-lung transplantation in the present patient who had multiple calcified emboli and severe right ventricular dysfunction.
During the differential diagnosis, cardiac neoplasias, especially myxomas and fibromas, are considerations, particularly if they are calcified, as are conditions involving infection or thrombosis.9) Due to the lack of histology, calcified atrial myxoma, calcified thrombi or other cardiac neoplasms should be also considered as a differential diagnosis of calcific mass of RV.
Echocardiography, and CT and MRI provide important information on the size and shape, attachment site, and pattern of movement of the calcified tumor. Myxomas usually have a short, broad-based attachment and are pedunculated, although calcification may develop in approximately 10% of myxomas. CT scans can detect intracardiac masses and define the extracardiac extension.10) CT scans can also detect even minute amounts of calcium, which facilitated detection of the calcific pulmonary embolism in the present case. MRI can provide accurate extension of the tumor and dysfunction of involved cardiac chambers.
We recommend utilizing a multimodality imaging approach to accurately characterize intracardiac masses and their complications.
Figures and Tables
Fig. 1
Transthoracic echocardiogram. Diffuse calcified mass affecting the tricuspid chordal apparatus and the free wall of the right ventricle (A), which caused significant tricuspid regurgitation (B). The systolic pulmonary artery pressure was about 65 mmHg considering a tricuspid jet velocity of 3.76 m/s (C) and dilated inferior vena cava of 2.78 cm (D). RA: right atrium, RV: right ventricle, LV: left ventricle, LA: left atrium.
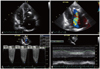
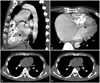
Fig. 2
Chest computed tomogram non-contrast imaging. The black arrowheads indicate calcified right ventricular amorphous tumor (A and B), and the white arrowheads indicate multiple pulmonary calcified emboli obstructing multiple pulmonary segmental arteries bilaterally (C and D).
References
1. Ho HH, Min JK, Lin F, Wong SC, Bergman G. Images in cardiovascular medicine. Calcified amorphous tumor of the heart. Circulation. 2008. 117:e171–e172.
2. Chaowalit N, Dearani JA, Edwards WD, Pellikka PA. Calcified right ventricular mass and pulmonary embolism in a previously healthy young woman. J Am Soc Echocardiogr. 2005. 18:275–277.

3. Tsuchihashi K, Nozawa A, Marusaki S, Moniwa N, Oh-numa Y, Kuno A, Takagi S, Takizawa H, Ura N, Shimamoto K. Mobile intracardiac calcinosis: a new risk of thromboembolism in patients with haemodialysed end stage renal disease. Heart. 1999. 82:638–640.

4. Reynolds C, Tazelaar HD, Edwards WD. Calcified amorphous tumor of the heart (cardiac CAT). Hum Pathol. 1997. 28:601–606.

5. Fealey ME, Edwards WD, Reynolds CA, Pellikka PA, Dearani JA. Recurrent cardiac calcific amorphous tumor: the CAT had a kitten. Cardiovasc Pathol. 2007. 16:115–118.

6. Morishima A, Sasahashi N, Ueyama K. [Calcified amorphous tumors with excision in hemodialysis patients: report of 2 cases]. Kyobu Geka. 2006. 59:851–854.
7. Gutiérrez-Barrios A, Muriel-Cueto P, Lancho-Novillo C, Sancho-Jaldón M. Calcified amorphous tumor of the heart. Rev Esp Cardiol. 2008. 61:892–893.

8. Habib A, Friedman PA, Cooper LT, Suleiman M, Asirvatham SJ. Cardiac calcified amorphous tumor in a patient presenting for ventricular tachycardia ablation: intracardiac echocardiogram diagnosis and management. J Interv Card Electrophysiol. 2010. 29:175–178.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download