Abstract
The heart and the brain, most oxygen-dependent organs, may be severely affected after carbon monoxide (CO) exposure. CO induced cardiotoxicity may occur as a consequence of moderate to severe CO poisoning, including angina attack, myocardial infarct, arrhythmias, and heart failure. We present a rare case of CO poisoning induced cardiomyopathy with left ventricular (LV) thrombus. It is thought that LV thrombus may have been caused severely decreased LV function with dyskinesis. After short-term anticoagulant therapy, echocardiography findings revealed complete recovery of LV dyskinesis and resolution of LV thrombus.
Acute carbon monoxide (CO) poisoning is an important clinical problem because of severe cardiovascular effects and a large proportion of fatal death.
Cardiac manifestations have been well demonstrated to occur in CO poisoning. However, its severity and duration has not yet known.1-3) Myocardial injury may occur as a consequence of moderate to severe CO poisoning, mostly manifested as elevated cardiac biomarkers and the changes of regional wall motion abnormality in transthoracic echocardiography (TTE).4) However, there is a little data regarding the relationship between CO poisoning and myocardial damage.3)
Left ventricular (LV) thrombus is not rare but well known complication of stress induced cardiomyopathy. However, its incidence and clinical significance in this stressful condition has not been well established.
To the best of our knowledge, the present report is the first Korean case of 66-year-old man with CO poisoning induced cardiomyopathy accompanied by LV thrombus.
A 66-year-old man was referred to the emergency room due to sudden onset of dyspnea with NYHA III-IV. He was using Briquet boiler. Physical examination revealed blood pressure 136/87 mmHg with a 1-year history of hypertension, body temperature 36.0℃, heart rate 97/min, and respiratory rate 28/min. The chest radiography showed mild cardiomegaly with pulmonary edema in both lung fields. The initial electrocardiographic findings revealed sinus tachycardia with a heart rate of approximately 130/min, T wave inversion in II, III, aVF and V3-V6 and diminished R waves in V1-V4. Laboratory findings on admission showed a marked elevation in the serum level of pro-brain natriuretic peptide of 18,699 pg/mL. Also, cardiac enzymes were elevated to a troponin T of 0.88 ng/mL and CK-MB of 9.7 ng/mL, and arterial blood gas reveals pH 7.41, PaCO2 26 mmHg, PaO2 61 mmHg, HCO3 16 mmol/L, SaO2 92%, and the fraction of carboxyhemoglobin 20.2% (reference range < 2%).
The TTE done on the same day revealed akinesis at the apex of LV and LV ejection fraction of less than 30% (Fig. 1). But the regional wall motion abnormalities extend beyond a single epicardial coronary distribution. Coronary angiography recommended but the patient refused, so the 128-channel multidetector computed tomography (MDCT) was conducted. MDCT revealed significant stenosis with severe calcification in three-all coronary arteries. He was transferred to the intensive care unit and provided oxygen for treatment of CO poisoning. Serial cardiac biomarkers were performed and normalized in several days. Follow-up TTE was done 7 days later, which showed a round thrombus (12 × 10 mm) at the apex of LV (Fig. 2A).
Anticoagulation was started at same time with heparin and warfarin. Partial thromboplastin time and subsequently International
Normalized Ratio were maintained at therapeutic level. However, sufficient anti-thrombotic agents could not be administered because active gastric ulcer bleeding suddenly occurred on the next day. Follow-up TTE performed on the twenty-one day of admission revealed the full recovery of the regional wall motion of LV and complete resolution of thrombus at the apex of LV (Fig. 2B).
The patient has been free of symptoms and there have been no clinical features of neurologic disorders or thromboembolic complications during the 6-months of clinical follow-up.
The cardiovascular manifestations of CO poisoning have been limited to case reports and presented variable type of myocardial dysfunction and injury. Until recently, the various mechanisms have been proposed.
Myocardial injury from CO poisoning results from tissue hypoxia as well as damage at the cellular level. The affinity of hemoglobin for CO is 200 to 250 times greater than its affinity for oxygen. This results in competitive inhibition of oxygen release due to a shift in the oxygen-hemoglobin dissociation curve, reduced oxygen delivery, and subsequent tissue hypoxia.2)5) Also, CO may bind to heme proteins, including myoglobin and cytochrome C oxidase.6) And, it may bind to cytochrome C oxidase, which is an enzyme in the mitochondrial electron-transport system chain that produces adenosine triphosphate by catalyzing the reduction of oxygen to water.7)
In a study by Satran et al.,2) different clinical patterns of myocardial injury were found. The pattern seen in younger patients with few coronary risk factors but severe CO poisoning was global LV dysfunction by TTE, which improved or resolved. This was consistent with stunned myocardium as a result of CO poisoning. The second pattern was seen in older patients with higher coronary risk factors. These patients had regional wall motion abnormalities suggesting that CO poisoning unmasks underlying coronary artery disease by creating supply/demand mismatch.2)3)
To our knowledge, this is the first Korean case of acute CO poisoning induced acute heart failure complicated with LV thrombus. However, stress induced cardiomyopathy associated with LV thrombus is not a rare but well known complication. De Gregorio et al.8) reported that LV thrombus formation results in about 2.5% of all the patients with documented stress induced cardiomyopathy. In general, LV thrombus disappears together with or before LV functional recovery.8)9) We experienced a case of acute CO poisoning induced transient LV dysfunction, which is apical ballooning form complicated with LV thrombus. The patient in this case has significant coronary artery disease, but he is fully recovered from segmental wall motion abnormality and LV thrombus within three weeks.
In conclusion, although the optimal therapy for stress induced cardiomyopathy with LV thrombus is still unknown, the use of anticoagulant therapy in the acute phase and until complete resolution of wall motion abnormalities appears to be appropriate in patients with apical thrombus.8)9) Because TTE can evaluate rapid change in cardiac condition, frequent follow-up using this technique is recommended for patients with stress induced cardiomyopathy, especially when anticoagulant therapy is difficult.
Figures and Tables
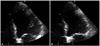 | Fig. 1The echocardiogram performed at admission reveals akinesis at the apex of LV and LV ejection fraction of less than 30%. A: End-diastole. B: End-systole. LV: left ventricle. |
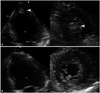 | Fig. 2A: Transthoracic echocardiogram demonstrates a 12 × 10 mm sized left ventricular thrombus at the apex on apical 4-chamber and short axis views (arrowhead). B: Complete resolution of the left ventricular thrombus is observed at the apex of LV on apical 4-chamber and short axis view after anticoagulation treatment. LV: left ventricle. |
References
1. Kalay N, Ozdogru I, Cetinkaya Y, Eryol NK, Dogan A, Gul I, Inanc T, Ikizceli I, Oguzhan A, Abaci A. Cardiovascular effects of carbon monoxide poisoning. Am J Cardiol. 2007. 99:322–324.

2. Satran D, Henry CR, Adkinson C, Nicholson CI, Bracha Y, Henry TD. Cardiovascular manifestations of moderate to severe carbon monoxide poisoning. J Am Coll Cardiol. 2005. 45:1513–1516.

3. Tucciarone M, Dileo PA, Castro ER, Guerrero M. Myocardial infarction secondary to carbon monoxide poisoning: an uncommon presentation of a common condition. Case report and review of the literature. Am J Ther. 2009. 16:462–465.

4. Jang WI, Park JH. Transient left ventricular systolic dysfunction associated with carbon monoxide toxicity. J Cardiovasc Ultrasound. 2010. 18:12–15.

6. Alonso JR, Cardellach F, López S, Casademont J, Miró O. Carbon monoxide specifically inhibits cytochrome c oxidase of human mitochondrial respiratory chain. Pharmacol Toxicol. 2003. 93:142–146.

7. Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr. 2008. 40:533–539.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download