Abstract
Background
Non-dippers were reported as showing different left atrial function, compared to dippers, but no study to date investigated the changes in the left atrial function according to the diurnal blood pressure pattern, using tissue Doppler and strain imaging.
Methods
Forty never treated hypertensive patients between 30 and 80 years of age were enrolled in this study. Patients were classified as non-dippers when, during night time, they had a blood pressure decrease of less than 10%. Strain of the left atrium was measured during late systole, and peak strain rates of the left atrium were measured during systole, early and late diastolic periods.
Results
The left atrial expansion index, left atrial active emptying volume and left atrial active emptying fraction were all significantly increased in non-dippers. They also had increased values of mean peak left atrial strain (dippers = 21.26 ± 4.23% vs. non-dippers = 24.91 ± 5.20%, p = 0.02), strain rate during reservoir (dippers = 1.29 ± 0.23 s-1 vs. non-dippers =1.52 ± 0.27 s-1, p = 0.01) and contractile period (dippers = -1.38 ± 0.24 s-1 vs. non-dippers = -1.68 ± 0.32 s-1, p < 0.01).
In hypertensive patients, blood pressure (BP) usually fluctuates during the 24-hour circadian rhythm. Thus, the mean blood pressure values are 10-20% lower during the night, compared to daytime measurement.1) This condition is called "the dipper" change. In contrast, non-dippers are defined as the patients without these diurnal fluctuations in blood pressure.2)3) The 24-hour ambulatory blood pressure (ABP) monitoring is widely used for the evaluation of diurnal fluctuation of BP. It has been demonstrated that ABP monitoring is a better predictor of cardiovascular complications than the conventional, spot measurement of blood pressure, and has become increasingly important for the management of hypertensive patients.2)
Non-dippers tend to develop increased incidence of target organ damage and are known to have poorer prognosis, due to higher incidence of cardiovascular events.3)4) In non-dippers, it has been demonstrated that target organ damages are due to the absence of night-time BP dipping, and are not the result of a higher overall BP load.5) Natriuretic peptides were reported to correlate with left ventricular (LV) diastolic function.6) Hypertensive patients were reported to have increased levels of cardiac natriuretic hormones and plasma atrial natriuretic peptide (ANP) levels were correlated with both the LV and left atrial (LA) abnormalities.7) Similarly, non-dippers are known to have more increased levels of cardiac natriuretic hormones.8)9)
During the cardiac cycle, the LA serves multiple functions, such as reservoir, conduit, active contractile chamber and suction source.10) It also modulates the LV filling through these various mechanical functions. Strain and strain rate are relatively newly introduced methods for myocardial function evaluation. Color Doppler tissue imaging (CDTI) can measure the strain and strain rate of the LA and its use in normal subjects has been validated.11) In many clinical conditions, including hypertension, diabetes and atrial fibrillation,12-15) CDTI is a useful tool to detect subclinical abnormalities of LA function.
Previous studies showed that diurnal fluctuation of BP could affect LA function, as evaluated by LA phasic volume.16) However, to the best of our knowledge, no study investigated to date the effect of diurnal fluctuation of BP on various LA functions by strain and strain rate using CDTI. It is also unknown whether the LA phasic functions could be affected when evaluated by deformation parameters, in a similar manner as in the case of volume parameters. Therefore, we investigated whether there is difference in LA function by BP diurnal variation, evaluated by CDTI in never-treated arterial hypertensive patients.
We recruited the subjects from patients who visited the outpatient clinic in Bucheon St. Mary's Hospital, Bucheon, South Korea for evaluation of the hypertension and underwent ABP monitoring. A total of 40 patients, aged between 30 and 80 years, and suffering from essential hypertension diagnosed for the first time, were enrolled in this study. Out of 40 patients, 20 were dippers and 20 were non-dippers. None of the patients was receiving antihypertensive medication at the initiation of the study. Patients with any of the following were excluded from the study: history of myocardial infarction; diabetes mellitus or taking diabetes medication; significant valvular disease; history of atrial fibrillation or other significant arrhythmia; serum creatinine ≥ 1.3 mg/dL; creatinine clearance rate ≤ 60 mL/min; and global or segmental systolic dysfunction on echocardiographic examination. This study was approved by the Institutional Review Board of Bucheon St. Mary's Hospital and all participants provided written consent, according to the Declaration of Helsinki.
After at least 5 min of rest in the sitting position, BP was measured during office visit, using a sphygmomanometer with the appropriate cuff size. Two BP values at least 5 min apart were measured and the mean BP value was used for analysis. BP was measured in both arms and the higher BP value was used for analysis in this study.
The ABP device (Tonoport V, GE Healthcare, Waukesha, WI, USA) was applied to the non-dominant arm of the patients included in this study. BP measurements were taken at 30-minute periods during daytime (i.e. between 06 : 00-22 : 00 h) and at 1-hour periods during nighttime (between 22 : 00-06 : 00 h). We also analyzed the BP values measured between 04 : 00-06 : 00 h, as BP at awakening time. If 20% or more of the measurements could not be taken, those patients were excluded or the procedure was repeated. The patients were instructed to perform their normal daily activities during the day and go to bed no later than 22 : 00 h. They were also instructed to stay in bed until 6 : 00 h. The individuals with daytime mean systolic BP/diastolic BP values equal to or higher than 135/85 mm Hg were defined as hypertensive. Patients with both systolic and diastolic BP decreases of 10% or more during nighttime were accepted as presenting the dipper status, whereas patients were classified as non-dipper if the blood pressure decrease during the night was less than 10%, either of the systolic or diastolic BP.
Echocardiography was performed with an ultrasound system (Vivid 7, GE Healthcare, Waukesha, WI, USA) with 2.5-MHz transducer. The M-Mode measurements included LV dimension, the diastolic LV septal and posterior thickness, determined in the parasternal long axis view. LV mass was calculated by the area-length method and corrected for the body surface area. The ejection fraction was calculated with the modified Simpson's method.17) From the pulsed Doppler echocardiography of transmitral velocities, peak E velocity, peak A velocity, the ratio between peak E and A velocities (E/A ratio), deceleration time and isovolumic relaxation time were acquired. The systolic S' velocity, early diastolic E' velocity and late diastolic A' velocity were measured, using Doppler tissue imaging. These measurements were acquired by placing the sample volume at the septal and lateral annulus, and recording at a sweep of 100 mm/s.
LA volumes were measured for evaluation of the LA phasic function. These volumes were, as follows: the LA maximal volume recorded at the onset of mitral opening; the LA minimal volume recorded at the onset of mitral closure; and the LA presystolic volume recorded just before the "p" wave on the ECG. All volumes were calculated by the biplane arealength method,17) and were indexed to the body surface area.
Then, the following parameters representing LA phasic functions were calculated, as previously described:18)
LA expansion index = (LA maximal volume - LA minimal volume) / LA minimal volume × 100
LA conduit volume = LV stroke volume - (LA maximal volume - LA minimal volume)
LA passive emptying volume = LA maximal volume - LA presystolic volume
LA passive emptying fraction = LA passive emptying volume / LA maximal volume × 100
LA active emptying volume = LA presystolic volume - LA minimal volume
LA active emptying fraction = LA active emptying volume / LA presystolic volume
LA ejection fraction = LA stroke volume / LA maximal volume × 100.
We also calculated the atrial fraction as the A wave velocity time integral divided by the total velocity time integral of the mitral inflow, as previously described.19)
The CDTI was obtained in the apical four and two chamber views, with the frame rate > 110 frames/sec. The narrowest image sector angle (usually 30° degrees) was used to achieve the maximum possible color Doppler frame rate, and attempts were made to align the atrial wall parallel to the Doppler beam. We also measured the peak LA strain during the late systole to evaluate the LA reservoir function (Fig. 1A). For evaluation of the LA phasic function, the strain rates of the LA were measured during the systolic, early and late diastolic periods, representing the reservoir, conduit and contractile functions of the LA, respectively (Fig. 1B). We also tracked the location of the region of interest to avoid falling into the fossa ovalis or LA appendage. All measurements were performed at the basal septal, lateral, inferior and anterior wall of the LA, from the apical 4- and 2-chamber views. Offline measurements were performed on the Echopac workstation version 6.1 (GE Healthcare, Waukesha, WI, USA). Each parameter was evaluated by averaging three to five measurements.
All data are expressed as the mean ± standard deviation. The independent t-test was used to assess the statistical difference between dippers and non-dippers. The chi-squared and Fisher's exact tests were used to evaluate the differences between categorical variables. Reliability was checked using Bland-Altman analyses to determine both the intra-observer and inter-observer variability. All data analyses were performed using the commercially available statistical analysis software package SAS version 11.0 (SAS Institute, Cary, NC, USA). p values less than 0.05 were considered as statistically significant.
Clinical characteristics, the levels of natriuretic peptide and BP values of the investigated patients were presented in Table 1. Age, gender distribution, the value of the body mass index and the lipid profile were statistically different between the groups. In contrast, the mean level of high sensitivity C-reactive protein was not significantly different. There was no statistically significant difference in the levels of ANP and N-terminal probrain natriuretic peptide (NT-proBNP), according to the diurnal BP pattern. There were no differences between the groups in the office, 24-hr average and daytime BP values. However, both systolic and diastolic BP values were significantly increased in the non-dippers group, during both nocturnal and awakening time.
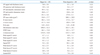
Conventional echocardiographic parameters were presented in Table 2. LV dimension, wall thickness and mass index were not significantly different between the two investigated groups. There were also no significant differences in the systolic and diastolic LV functions, according to the diurnal BP pattern.
LA phasic volumes and other parameters representing the LA function were shown in Table 3. LA maximal volume and LA volume at the onset of the atrial systole were significantly increased in the non-dipper group. Although there was no difference in both LA passive emptying volume and fraction between the two groups, the LA expansion index, LA active emptying volume and LA active emptying fraction were significantly increased in the non-dippers group. In contrast, the LA conduit volume was increased in the dippers group (dippers = 21.43 ± 6.51 mL/m2 vs non-dippers = 17.05 ± 5.80 mL/m2, p = 0.03).
Table 4 shows the peak strain value of the LA measured during the reservoir period. Although there was no significant difference between the groups in the segmentally evaluated values, the averaged values showed that the peak strain of the LA was significantly increased in the non-dippers group (dippers = 21.26 ± 4.23% vs non-dippers = 24.91 ± 5.20%, p = 0.02). The strain rates of the LA were also significantly different between dippers and non-dippers. Thus, the strain rates measured during the reservoir and contractile periods showed differences between the groups. In contrast, the difference in strain rate measured during the conduit period was not statistically significant between the two groups (Table 4). When comparing the natriuretic peptide levels and the deformation parameters, we found only weak relationship between the values of the strain measured at the septum and the serum value of ANP.
Bland-Altman plots were demonstrated in Fig. 2. LA strain and strain rates showed good agreement between intra- and inter-observer variability.
In this study, we demonstrated that patients in the nevertreated non-dippers group had exaggerated reservoir and booster pump functions of the LA. Many volumetric parameters of the LA showed differences between the dipper and nondipper groups. Thus, the LA maximal volume index, LA volume at the onset of the atrial systole, LA expansion index, LA active emptying volume, LA active emptying fraction and the LA ejection fraction were all significantly increased in the nondippers group. These findings were consistent with results from a previous study performed by Aydin et al.16) In addition, in this study, we evaluated the LA function using the tissue Doppler and strain imaging methods which were relatively newly introduced. Although there were no differences between the two investigated groups in tissue velocities, both strain and strain rate of the LA showed significant differences between dippers and non-dippers. Thus, the peak LA strain measured during the reservoir period was significantly increased in the non-dippers group. The peak LA strain rates evaluated during both reservoir and contractile periods were also increased in the non-dipper patients. In contrast, the deformation parameters of the LA were not correlated with the serum levels of natriuretic peptides (i.e. NT-proBNP and ANP), demonstrating cardiac muscle stretching.
In hypertensive patients, LV hypertrophy occurs and results in diastolic dysfunction. Systemic hypertension is the leading cause of left ventricular hypertrophy and impaired left ventricular diastolic filling. Enlargement of the left atrium might be attributed to the impairment of blood flow from left atrium to left ventricle due to increased left ventricular stiffness.20) The LA functions as a reservoir, passive conduit and booster pump, according to various cardiac cycles, and acts as a modulator of the diastolic function of the LV.10) LA reservoir functions occur during the ventricular systole and the passive conduit functions occur in the early diastole. The reservoir function of the LA is affected when there is acute LV regional ischemia and it is determined by the systolic function of the LV, as well as the relaxation period of the LA.20) The LA also acts as an active contractile chamber that augments the filling of the left ventricle during the late diastole, and as a suction source that refills itself in the early systole.20) The active atrial emptying increases to maintain sufficient output in case of systemic hypertension, where LV diastolic function deteriorates.21) In our study non-dipper patients showed significantly increased LA strain rate during the late diastole, representing booster pump function of the LA.
The function of the LA in hypertensive patients is different from the normal subjects. Thus, it has been demonstrated that both maximal and presystolic volumes of the LA increase, even in the mild hypertension patients. The LA active emptying volume, i.e. indicator of the contractile function of the LA, was also found elevated in the same report.22) Another study, using the strain and strain rate of the LA, showed that the conduit function was also changed in hypertensive patients.12)
Usually, the blood pressure has diurnal fluctuation, but the non-dipper hypertensive patients did not show the normal circadian rhythm of BP. These patients were known to have increased incidence of target organ damage.3)23) Similarly, the non-dippers were known to have increased incidence of left ventricular hypertrophy, LV diastolic dysfunction and atrial rhythm disturbances, compared to the dipper patients.24-26) Non-dippers were also known to have elevated serum levels of natriuretic peptide.8) It has been shown that, in time, even normotensive subjects could develop target organ damage or elevated levels of natriuretic peptide, if the circadian BP patterns were lost.27)28) One previous study using LA phasic volumes showed that both reservoir and booster pump functions of the LA were increased in non-dipper patients.16) However, no study evaluated the effects of the circadian BP pattern on the LA function measured by tissue Doppler or strain imaging methods.
In this study, we showed that both the reservoir and booster pump functions of the LA were increased in non-dippers, when evaluated with strain and strain rate measured by CDTI. These results were consistent with results from measurement of the LA phasic volumes, as reported previously.16) Using strain and strain rate for the evaluation of the LA function has some benefits. First, one study investigating the relationship between the conventional and deformation parameters measured using CDTI was published for the evaluation of the LA function.11) Thus, both the utility and reproducibility of this method were validated. Second, many clinical conditions, such as hypertension, diabetes mellitus and atrial fibrillation, were studied using this technique and the clinical implications developed from previous studies.13-15)22) Third, all the parameters representing various functions of the LA can be measured from one imaging. This benefit is especially important because the evaluation of phasic LA volumes requires multiple measurements and multistage calculations, which may result in many errors. Recently, evaluation of the LA function using the two-dimensional speckle tracking technique also validated its clinical significance in several clinical situations.29) Nevertheless, thin LA walls and different values of LA parameters according to the measured location make this technique less feasible,30) and a recently published consensus suggested that current speckle tracking echocardiography measurements were not ready for clinical use for evaluation of the LA function.31)
This study also has some limitations. First, the measurement of both strain and strain rate of the LA using CDTI requires off-line analysis using the specified workstation and software, and is therefore time consuming. This method is also technically demanding, because only a well aligned images and narrow sample area are eligible for analysis because of its angle and noise dependency. And the values of strain and strain rate of the LA are different according to the segments and there are no generally accepted normal values.10)14) Similarly, the normal reference values for LA strain and strain rate were only evaluated in a relatively small number of patients, so currently there is no widely accepted consensus about normal values for the LA strain and strain rates. Second, the sample size in the present study was relatively small. This limitation can be the cause of only the weak relationship that we found between deformation parameters and volumetric parameters. But both of the parameters consistently showed which components of the LA function were affected by diurnal BP variation.
In conclusion, various LA functions showed differences in the never-treated non-dipper hypertensive patients, compared to dipper patients. The function of the LA was altered irrespective of the LV mass index or other echocardiographic parameters routinely measured for the evaluation of both systolic and diastolic functions of the left ventricle. Thus, the LA function, which modulates the diastolic phase, can be responsible for both functional and morphologic cardiac changes observed in the non-dipper patients. Both strain and strain rate of the LA, measured using CDTI, can be useful and simple parameters for the evaluation of the subtle changes and various LA functions in hypertensive patients.
Figures and Tables
Fig. 1
A: Arrow indicates peak left atrial strain during the late systole. B: Arrows indicate peak left atrial strain rate during systole, early and late diastole.
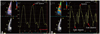
Table 1
Clinical characteristics and blood pressure of patients with dipper and non-dipper type of hypertension
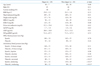
Table 2
Conventional echocardiographic parameters according to the diurnal blood pressure fluctuation
Acknowledgements
This work was supported by a research grant of the Korean Society of Echocardiography.
References
1. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ;. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003. 289:2560–2572.

2. Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A, Santuci C, Reboldi G. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994. 24:793–801.

3. Pickering TG. The clinical significance of diurnal blood pressure variations. Dippers and nondippers. Circulation. 1990. 81:700–702.

5. Cuspidi C, Meani S, Valerio C, Fusi V, Catini E, Sala C, Zanchetti A. Ambulatory blood pressure, target organ damage and left atrial size in never-treated essential hypertensive individuals. J Hypertens. 2005. 23:1589–1595.

6. Kim WS, Park SH. Correlation between N-Terminal Pro-Brain Natriuretic Peptide and Doppler Echocardiographic Parameters of Left Ventricular Filling Pressure in Atrial Fibrillation. J Cardiovasc Ultrasound. 2011. 19:26–31.

7. Wambach G, Bönner G, Stimpel M, Kaufmann W. Relationship between plasma atrial natriuretic peptide and left atrial and left ventricular involvement in essential hypertension. J Hypertens. 1988. 6:573–577.

8. Nakatsu T, Shinohata R, Mashima K, Yuki Y, Nishitani A, Toyonaga S, Ogawa H, Hirohata S, Usui S, Kusachi S. Use of plasma Btype natriuretic peptide level to identify asymptomatic hypertensive patients with abnormal diurnal blood pressure variation profiles: nondippers, extreme dippers, and risers. Hypertens Res. 2007. 30:651–658.

9. Nyström F, Malmqvist K, Lind L, Kahan T. Nurse-recorded clinic and ambulatory blood pressures correlate equally well with left ventricular mass and carotid intima-media thickness. J Intern Med. 2005. 257:514–522.

10. Leung DY, Boyd A, Ng AA, Chi C, Thomas L. Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J. 2008. 156:1056–1064.

11. Sirbu C, Herbots L, D'hooge J, Claus P, Marciniak A, Langeland T, Bijnens B, Rademakers FE, Sutherland GR. Feasibility of strain and strain rate imaging for the assessment of regional left atrial deformation: a study in normal subjects. Eur J Echocardiogr. 2006. 7:199–208.

12. Eshoo S, Boyd AC, Ross DL, Marwick TH, Thomas L. Strain rate evaluation of phasic atrial function in hypertension. Heart. 2009. 95:1184–1191.

13. Muranaka A, Yuda S, Tsuchihashi K, Hashimoto A, Nakata T, Miura T, Tsuzuki M, Wakabayashi C, Watanabe N, Shimamoto K. Quantitative assessment of left ventricular and left atrial functions by strain rate imaging in diabetic patients with and without hypertension. Echocardiography. 2009. 26:262–271.

14. Di Salvo G, Caso P, Lo Piccolo R, Fusco A, Martiniello AR, Russo MG, D'Onofrio A, Severino S, Calabró P, Pacileo G, Mininni N, Calabró R. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation: a color Doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation. 2005. 112:387–395.

15. Schneider C, Malisius R, Krause K, Lampe F, Bahlmann E, Boczor S, Antz M, Ernst S, Kuck KH. Strain rate imaging for functional quantification of the left atrium: atrial deformation predicts the maintenance of sinus rhythm after catheter ablation of atrial fibrillation. Eur Heart J. 2008. 29:1397–1409.

16. Aydin M, Ozeren A, Bilge M, Dursun A, Cam F, Elbey MA. Effects of dipper and non-dipper status of essential hypertension on left atrial mechanical functions. Int J Cardiol. 2004. 96:419–424.

17. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Chamber Quantification Writing Group. American Society of Echocardiography's Guidelines and Standards Committee. European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005. 18:1440–1463.

18. Toutouzas K, Trikas A, Pitsavos C, Barbetseas J, Androulakis A, Stefanadis C, Toutouzas P. Echocardiographic features of left atrium in elite male athletes. Am J Cardiol. 1996. 78:1314–1317.

19. Erol MK, Yilmaz M, Acikel M, Karakelleoglu S. Left atrial mechanical function in patients with essential hypertension. Acta Cardiol. 2002. 57:323–327.
20. Barbier P, Solomon SB, Schiller NB, Glantz SA. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation. 1999. 100:427–436.

21. Matsuda M, Matsuda Y. Mechanism of left atrial enlargement related to ventricular diastolic impairment in hypertension. Clin Cardiol. 1996. 19:954–959.

22. Eshoo S, Ross DL, Thomas L. Impact of mild hypertension on left atrial size and function. Circ Cardiovasc Imaging. 2009. 2:93–99.

23. Pickering TG, James GD. Ambulatory blood pressure and prognosis. J Hypertens Suppl. 1994. 12:S29–S33.

24. Kim BK, Lim YH, Lee HT, Lee JU, Kim KS, Kim SG, Kim JH, Lim HK, Shin J. Non-Dipper Pattern is a Determinant of the Inappropriateness of Left Ventricular Mass in Essential Hypertensive Patients. Korean Circ J. 2011. 41:191–197.

25. Seo HS, Kang TS, Park S, Choi EY, Ko YG, Choi D, Ha J, Rim SJ, Chung N. Non-dippers are associated with adverse cardiac remodeling and dysfunction (R1). Int J Cardiol. 2006. 112:171–177.

26. Ermis N, Acikgoz N, Cuglan B, Cansel M, Yagmur J, Tasolar H, Barutcu I, Pekdemir H, Ozdemir R. Comparison of atrial electromechanical coupling interval and P-wave dispersion in non-dipper versus dipper hypertensive subjects. Blood Press. 2010. 20:60–66.

27. Soylu A, Yazici M, Duzenli MA, Tokac M, Ozdemir K, Gok H. Relation between abnormalities in circadian blood pressure rhythm and target organ damage in normotensives. Circ J. 2009. 73:899–904.

28. Hoshide S, Kario K, Hoshide Y, Umeda Y, Hashimoto T, Kunii O, Ojima T, Shimada K. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community-dwelling normotensives. Am J Hypertens. 2003. 16:434–438.

29. Saha SK, Anderson PL, Caracciolo G, Kiotsekoglou A, Wilansky S, Govind S, Mori N, Sengupta PP. Global left atrial strain correlates with CHADS2 risk score in patients with atrial fibrillation. J Am Soc Echocardiogr. 2011. 24:506–512.

30. Vianna-Pinton R, Moreno CA, Baxter CM, Lee KS, Tsang TS, Appleton CP. Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr. 2009. 22:299–305.

31. Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011. 24:277–313.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download