Introduction
Microbubble contrast agents and specific imaging technologies designed to suppress tissue "noise" while enhancing contrast "signal" are currently used during echocardiography to improve endocardial border delineation, allowing improved visualization of myocardial segments, providing better confidence and accuracy in the assessment of wall motion, as well as decreasing inter- and intra-observer variability. Accurate evaluation of wall motion is particularly important when echocardiography is used to detect acute coronary syndromes, and during stress echocardiography. Contrast agents have been shown to enhance the evaluation of the left ventricular apex -improving the visualization of apical hypertrophy, masses, aneurysm, and cardiomyopathies like isolated non-compaction. Contrast echocardiography can now also be used to assess myocardial perfusion, to quantify myocardial blood flow, microvascular integrity and viability. These applications use microbubble agents which are free-flowing within the microvasculature.
The next frontiers in contrast ultrasonography are targeted imaging, and using microbubbles for therapeutic purposes. Targeted imaging is the detection of specific components of cardiovascular disease in vivo, with microbubbles which may non-specifically attach to diseased endothelial cells, or with microbubbles which have been specifically designed to detect a pathologic process. Therapeutic applications of contrast ultrasonography include the use of microbubbles to enhance delivery of agents (like drugs, genes, growth factors, etc.) to the endothelium or perivascular cells. This review will discuss differences in contrast agents used for current applications versus targeted imaging, technical considerations required to achieve site-specific imaging, and potential applications of this technology. The potential for contrast ultrasonography to enhance drug and gene delivery to tissue will also be discussed.
Microbubble Contrast Agents
The contrast agents that are used during echocardiography are composed of microbubbles which contain a high-molecular weight gas (most commonly perfluorocarbons), encapsulated within a thin shell. Perfluorocarbons have low diffusibility and solubility in the circulation, which allows them to enhance the systemic circulation after venous injection.
Gaseous microbubbles produce strong ultrasound backscatter signals because they are 4-5 times more compressible than water or tissue. During the alternate pressure cycles of ultrasound, microbubbles undergo volumetric oscillation in the acoustic field whereby they are compressed during the pressure peaks and expand during the troughs.1)2) Radial oscillation of the microbubbles results in the generation of strong backscattered acoustic signals that produce contrast enhancement or opacification on ultrasound imaging. Although larger microbubbles produce much greater acoustic signals, their maximum size is limited by the diameter of the pulmonary capillaries, which is approximately 5 µm. Current contrast agents are approximately 2-3 µm in size.
Microbubble contrast agents possess a number of unique properties that distinguish them from tracers used with other non-invasive imaging technologies. The microbubbles remain entirely intravascular, unlike nuclear tracers such as thallium or 99mTc-sestamibi which are extracted by myocytes, or radiologic contrast agents for computed tomography or gadolinium tracers for magnetic resonance imaging that diffuse into the interstitial space. The microbubbles are hemodynamically inert, so they do not affect local or systemic blood flow.3) By comparing the transit of microbubbles with Tc-labeled red blood cells, or by direct visualization of fluorescently labeled microbubbles and red blood cells, the in vivo myocardial kinetics and rheology of microbubbles have been shown to be very close to that of red blood cells.4-6)
The currently available microbubbles have no interactions with the endothelium or its glycocalyx to affect their transit.7-9) For the most part, microbubble shells minimize their interaction with cellular elements. For example, most lipid shells are essentially neutral in charge, and some contain polyethylene glycol, which prevents interactions with serum proteins and cells.10)11) These measures extend the intravascular lifespan of bubbles by reducing cellular uptake in reticuloendothelial organs.
All the above properties allow microbubble contrast agents to pass freely through the microcirculation, and they do not lodge within the microcirculation. Fig. 1 shows sequential still frames obtained from an animal experiment where Albunex (an air-filled first generation microbubble agent with a denatured albumin shell) was injected directly into the left anterior descending coronary artery (LAD). As the microbubbles wash in and out of the perfusion bed of the LAD (Fig. 1B, C and D) (9 o'clock to 1 o'clock), there is an increase in myocardial contrast enhancement (Fig. 1B) followed by wash out (Fig. 1C and D). The change in video intensity in the myocardium as the microbubbles flow in and out of the microcirculation is shown in Fig. 1E, and the time-intensity curve can be fitted to a gamma-variate function. Using classic indicator-dilution curve theory, it was actually shown that these curves could be used to quantify myocardial blood flow or volume.12)13)
However, in experiments where the heart had been exposed to cardioplegia, a very different time-intensity curve was seen. Rather than the usual wash-in and wash out, there was persistent contrast enhancement in the myocardium (Fig. 1F). This means that the microbubbles were no longer freely flowing, but were instead persisting in the microcirculation.
Lindner et al.14) subsequently showed that the cardioplegia had caused ischemia-reperfusion injury, which resulted in up-regulation of inflammatory proteins and white cell activation. The microbubbles were "sticking" to areas of inflammation. Subsequent studies with Optison (a second generation perfluoropropane-filled microbubble with a denatured albumin shell) showed that adhesion was occurring through non-specific interactions between MAC-1 and the denatured albumin shell, or through binding with complement components in the case of phospholipid shelled microbbles (like Definity). These experiments showed for the first time that contrast ultrasound could be used to detect molecular events within the circulation non-invasively.
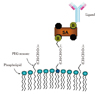
In order to accomplish true "targeted imaging", which should allow a user to detect a particular molecular or cellular process of interest, non-specific binding is insufficient. Thus, microbubbles targeted to attach to specific proteins can be produced. A spacer arm like polyethylene glycol can be conjugated to the surface of the microbubble, and then an avidin-biotin link can be used to attach a disease-specific ligand such as a monoclonal antibody, peptides, and so forth, to the arm. Fig. 2 shows an example of this type of construct on the surface of a microbubble. More than 60,000 ligands can be attached to the surface of each microbubble in this fashion.15)
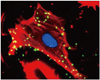
Fig. 3 shows fluorescent microscopy of microbubbles conjugated with an antibody directed against intercellular adhesion molecule-1 (ICAM-1), a protein that appears on the endothelial cell surface in inflammation. When there is inflammation, ligands are up-regulated on the endothelial cell surface of venules. White blood cells will then be captured and will roll on the blood vessel wall and then eventually move through the vessel wall (diapedesis) into tissue where they participate in the inflammatory process. In Fig. 3, "targeted microbubbles" (green) are shown abundantly attached to activated endothelial cells overexpressing ICAM-1 in vitro.16)
Detection of Targeted Microbubbles in vivo
At high acoustic pressures, exaggerated microbubble oscillation leads to microbubble destruction by one of several mechanisms. Microbubble compression can increase surface tension and cause outward diffusion of the microbubble gas during each high pressure ultrasound cycle, resulting in a gradual reduction in microbubble volume. Exaggerated radial oscillations can result in permanent defects in the microbubble shell, which results in rapid loss of gas from the microbubbles. Finally, abrupt fragmentation of microbubbles have been visualized with only a single high energy ultrasound pulse.2)17)18) Microbubble destruction results in the generation of strong harmonic signals which can be used to separate microbubbles from tissue noise, but to detect microbubbles which are attached to the endothelial surface, only a single pulse of high energy ultrasound could be used since all the microbubbles within the sound field would subsequently be eliminated. Therefore, although such an imaging technique could be used for in vitro proof-of-principle studies and animal experiments, it would not be feasible in a clinical setting.
When the mechanical index of ultrasound is reduced to very low levels (-0.1), and the frequency of ultrasound approximates the resonant frequency of the microbubble,19)20) non-linear microbubble oscillation can be induced during insonation without microbubble destruction, which still results in the production of unique harmonic signals. Tissue backscatter signals, on the other hand, contain very low amplitude harmonic signals at such a low mechanical index, making the separation of microbubble signals from tissue noise possible. Because imaging is being performed at a low power that prevents microbubble destruction - high frame rates and continuous imaging can be used which offers multiple advantages for targeted imaging. For example, a user could localize the correct scanning plane without destroying the targeted microbubbles and degrading the signal. Targeted microbubbles attached to a cell surface, and even microbubbles which have been phagocytosed into leukocytes continue to remain acoustically active.21-23)
In order to minimize interference from freely circulating microbubbles, targeted microbubbles which are not attached to the endothelial cell surface are given time to clear from the circulation before imaging is performed. In general, a delay of approximately 10 min is adequate to allow free microbubbles to be cleared by the reticuloendothelial system, at which time imaging should detect only targeted agent retained in diseased tissue.15)
Applications of Molecular Imaging
Inflammatory imaging
2D echocardiography can be used to evaluate wall thickening, which is closely dependent on resting myocardial blood flow (MBF). Because myocardial contractility is a major determinant of myocardial oxygen consumption, reductions in resting MBF are followed within seconds by the development of a wall thickening abnormality.24) Furthermore, ischemic myocardial segments with low resting flow will demonstrate resting perfusion defects. Thus, myocardial contrast echocardiography (MCE) can determine whether patients with acute chest pain are suffering from an acute coronary syndrome (ACS). In a prospective study of 114 patients presenting to the emergency room with "suspected cardiac chest pain", myocardial perfusion defects demonstrated 77% sensitivity for the detection of ACS compared to 28% and 34% respectively with ECG and troponin while maintaining similar specificity (89-96%).25) Abnormal myocardial perfusion was the only independent variable for diagnosing an ACS (odds ratio = 87, p < 0.001).
The short and long-term prognostic significance of MCE has also been shown in chest pain patients.26) Patients with abnormal perfusion were 2.5-fold more likely to have non-fatal myocardial infarction or cardiac death, but those with both abnormal regional function and myocardial perfusion were 14.3-fold (p < 0.001) more likely to have events - demonstrating the incremental benefit of combined wall thickening and perfusion data over regional function alone in these patients.
The diagnostic and predictive value of contrast echocardiography in acute chest pain is limited by a number of factors, however. When a patient has only ischemia but no infarction, wall thickening abnormalities (stunning) resolves over time - so perfusion and function may both have returned to normal if a patient presents late after their insult. In patients with prior infarction, it may be difficult to determine if a wall thickening abnormality is due to acute ischemia, or to remote infarction. In such cases, targeted imaging to identify recent ischemia-reperfusion injury (ischemic memory imaging) may be very valuable.
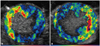
In one study, Ley et al.26) chose to detect the presence of P-selectin upregulation after ischemia. P-selectin is an endothelial adhesion molecule which is transported to the endothelial cell surface rapidly after an inflammatory stimulus, where it participates in leukocyte capture and rolling on the venular surface.26) The presence of P-selectin can persist for many hours after the initial injury. The anterior myocardium of mice were subjected to 10 min of ischemia followed by 45 min of reperfusion to allow recovery of resting function. Biotinylated microbubbles conjugated with a monoclonal antibody targeted against P-selectin were administered after reperfusion and showed selective retention and contrast enhancement of the post-ischemic anterior wall at a time when both myocardial perfusion and wall thickening had normalized.27) In Fig. 4, P-selectin targeted microbubbles were administered in open-chest dogs which had been subjected to 90 min of ischemia followed by reperfusion. The area of contrast enhancement has been color-coded so that green to yellow to red reflect greater signal intensity. Panels A and B demonstrate separate animals with left anterior descending (Fig. 4A), and left circumflex (Fig. 4B) territory ischemia followed by 60 min of reperfusion. Intense retention of targeted microbubbles resulting in bright enhancement is seen in the reperfused beds of the respective coronary arteries.28) Yan et al.29) evaluated the ability of molecular ultrasound to detect the presence of ICAM-1, which is upregulated on the venular endothelial surface later than P-selectin, but persists for much longer - with peak expression at 24 h after reperfusion. Accordingly, biotinylated microbubbles conjugated with a monoclonal antibody targeted against ICAM-1 were administered at 1, 8 and 24 h after a brief 15 min ischemic insult in mice. Selective enhancement of the post-ischemic anterior myocardium was demonstrated at the 8 and 24 h time points using targeted microbubbles. At 1 h, though, anti-ICAM-1 targeted microbubbles did not cause greater enhancement than microbubbles conjugated with a control antibody.29) These experiments demonstrate the dramatic ability of targeted imaging to identify myocardium which has been ischemic even up to 24 h after the insult. The endothelial target of choice will vary depending on the interval between the ischemic insult and the time of imaging (P-selectin early and ICAM-1 late), which highlights the need for a user to have good understanding of molecular biology to take full advantage of this technology.
Another condition characterized by inflammation is acute heart transplant rejection. Rats that had undergone heterotopic cardiac transplantation with and without acute rejection were administered microbubbles targeted to ICAM-1. Persistent diffuse myocardial contrast enhancement was noted in rejecting allografts after administration of targeted microbubbles, while no enhancement was seen after administration of control microbubbles.30)
Detection of early atherosclerosis
Atheromatous lesions are sites of endothelial dysfunction, intimal thickening, and there is overexpression of adhesion molecules to mediate leukocyte infiltration into plaque.31) The ability to detect early atherosclerosis in vivo, prior to the development of a significantly stenotic lesion or a cardiovascular event, could potentially be used to identify high risk individuals in need of aggressive treatment and risk-factor modification for primary prevention. The ability of microbubbles labeled with vascular cell adhesion molecule-1 (VCAM-1) to identify early atherosclerotic lesions in the aorta of apo-E knockout mouse has now been demonstrated.32) The degree of contrast enhancement was related to the extent of plaque, so targeted microbubbles can not only detect the presence of early atheromatous lesions, but also quantify the degree of associated inflammation.32)
Imaging of angiogenesis
The development of new blood vessels can be due to remodeling of new or pre-existing coronary collaterals (arteriogenesis), to the formation of new blood vessels from endothelial progenitor cells (vasculogenesis), or to the formation of new blood vessels from pre-existing vessels (angiogenesis). Contrast ultrasound could play 2 roles in angiogenesis - targeted microbubbles could be used to monitor the presence and development of new blood vessels, and microbubbles could also be used to deliver drugs or genes to promote and maintain an angiogenic response. This latter role will be discussed further below.
Imaging angiogenesis has utilized agents that have been targeted against integrins (such as αv -integrins like αvβ3 or αvβ5), against growth factors or their receptors (e.g. vascular endothelial growth factor or VEGF2 receptors), and against endothelial cell markers (such as VCAM-1).
The angiogenic models that have been used for imaging include tumor neovessels, matrigel plugs impregnated with fibroblast growth factor-2 (FGF-2) to stimulate angiogenesis development from surrounding vessels, and ischemia models. For example, microbubbles bearing antibodies to VEGF2 receptor have been shown to enhance murine breast cancer models,33) contrast agents conjugated to small peptides which have strong affinity integrins like the arginine-glycine-aspartate (RGD) containing disintegrin echistatin have been used to enhance human squamous cell carcinoma implanted in nude mice,34) or arginine-arginine-leucine peptide has been used to detect Clone C and PC3 tumors in mice.35) The microbubbles selectively adhere to tumor-derived rather normal endothelium, which suggests that targeted contrast ultrasound has the potential to be used to characterize tumor angiogenesis, and subsequently to monitor antitumor or antiangiogenic therapies.
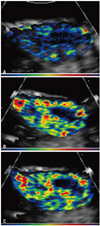
Fig. 5 shows the ability of targeted agents to detect neovessels in a murine model using a Matrigel plug impregnated with FGF-2 which stimulates angiogenesis from surrounding vessels. The microbubbles were conjugated with a monoclonal antibody against αv - integrins and produced significant enhancement of angiogenesis that had developed around the periphery of the plug (Fig. 5B).36) Similar success was shown using microbubbles conjugated to RGD peptide containing disintegrin echistatin (Fig. 5C).36)37) Conversely, no signal enhancement was noted when imaging was performed using a non-specific isotype antibody (Fig. 5A).36)
Targeted microbubbles and ischemia models have been used to evaluate the role of angiogenesis in improving perfusion to ischemic tissue, and have also been used to assess the role of growth factors on enhancing angiogenesis. Using a chronic hind-limb ischemia model in the rat of unilateral iliac artery ligation, the natural arteriogenic response to ischemia was demonstrated using a microbubble conjugated to a RGD-peptide, and the augmentation in neovascularization that occurred when FGF-2 was administered to the ischemic hindlimb was shown.38) Behm et al.39) then used microbubbles targeted to neutrophils, monocyte α5 - integrins, and VCAM-1 at different time points after iliac ligation. They demonstrated that early after ligation, all 3 components were present, followed by a precipitous decline in neutrophil signal after 2-4 days after ligation, with persistence of monocyte and VCAM signal until day 7. Improvement in tissue perfusion increased much more slowly, not peaking until day 21 post-ligation.39) This study demonstrated the power of targeted contrast ultrasound to evaluate separate factors which contribute to angiogenesis both functionally and temporally.
Therapeutic applications of contrast ultrasound
Gene therapy is limited by lack of a safe and effective method for gene delivery. Viral vectors have potential for immunogenic and cytotoxic effects. Plasmid delivery is safe, but have low transfection rates even with direct injection.
Microbubbles can potentially be used for therapeutic purposes, by enhancing the delivery of genes or drugs to specific targets. Microbubble destruction appears to be an important aspect of this method, especially when the microbubbles are destroyed in close proximity to the endothelial cell surface such as when the microbubbles attached to endothelial cells, or lodged in small arterioles.40) The mechanism by which microbubbles enhance gene/drug uptake is not entirely clear, but it is possible that temporary cellular membrane poration can be produced by shell fragmentation, which could enhance the uptake of DNA into perivascular cells.40-42) Shell implantation could also be enhanced through the generation of high-velocity pressure jets, heat or free radicals which are produced in the vicinity of the endothelial surface by microbubble destruction.
Microbubbles bearing viral vectors, plasmid DNA and antisense oligonucleotides have been used to enhance delivery to tissues. Fig. 5 shows extravascular deposits of plasmid containing luciferase cDNA which was charge-coupled to the surface of cationic microbubbles under fluorescent microscopy.40) The fluorescent DNA can be seen in the perivascular muscle adjacent to microvessels. These experiments demonstrated that no DNA deposition occurred in the absence of ultrasound. Furthermore, there was significantly greater DNA deposition following intra-arterial administration of microbubbles compared to intravenous, because the microbubbles with the former would not be subjected to pulmonary filtering and would become lodged within very small arterioles and capillaries. Despite the fact that microbubbles were being destroyed in close proximity to the endothelial surface, the vast majority of deposition events (85-90%) occurred without visible vascular ruptures or hemorrhage.40) The most efficient deposition occurred with intra-arterial injection, and with high power ultrasound exposure. If either component was missing, there was much less transfection (Fig. 6).
Summary and Conclusions
Future applications of contrast ultrasound have expanded the use of microbubbles beyond enhancement of the left ventricular cavity, improvement in the delineation of cardiac structures and systemic Doppler signals, and even myocardial perfusion imaging. As discussed in this review, microbubbles can now be used to define molecular and cellular pathology within the vascular space. The ability to identify inflammation, angiogenic vessels, and early atheroma are only some examples of how this technology can be applied, and are likely to augment the diagnostic utility of ultrasound not only for cardiac conditions, but for diseases affecting other organs which can be imaged using ultrasound. Targeted imaging using ultrasound certainly has the potential to enhance the ability to make earlier diagnoses, and to guide therapy. Furthermore, microbubbles may be used for therapeutic purposes, and enhance the delivery of drugs, genes, or other compounds directly to a site of greatest need. Certainly much more research, validation, and training are needed before these technologies will be incorporated into clinical practice - but the future of contrast ultrasound holds great promise and excitement.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download