Abstract
The left ventricle twists in systole storing potential energy and untwists (recoils) in diastole releasing the energy. Twist aids left ventricular ejection and untwist aids relaxation and ventricular filling. Therefore, rotation and torsion are important in cardiac mechanics. However, the methodology of their investigations is limited to invasive techniques or magnetic resonance imaging. With the advent of speckle tracking echocardiography, however, rotation and torsion (twist) become familiar to echocardiographers. In this review, I outline the mechanism and influencing factors of rotation and torsion with the anticipation of the routine use of these measurements in clinical practice.
It has been known for a long time that the heart makes rotation along its long-axis and a wringing (twisting) motion. Many investigators have made use of various different techniques to measure this twist motion and to attempt to explain its significance. Such techniques include embedding radiopaque markers in the myocardium and observing their movements through biplane cine angiography,1) and making observations with sonomicrometry in animal hearts.2) Many of these techniques are invasive, and thus unsuitable for observing the hearts of human subjects. Since the 1990s, however, techniques employing magnetic resonance imaging (MRI) tagging have seen extensive use.3)4) While MRI has greatly furthered our understanding of the twist motion of the human heart and thereby cardiac physiology, the need for large installations and the significant costs incurred mean that the range of applications is severely restricted.
Echocardiography is a noninvasive technique that can be carried out at the bedside, and therefore has become the optimal tool for studies on cardiac mechanics of diseased and healthy hearts. Thus, a number of studies on cardiac function, hemodynamics and cardiac physiology have been performed using echocardiography. There have been also many attempts to evaluate cardiac rotation and twist by echocardiography. However, the best that can be said with conventional 2-dimensional echocardiography is that the short-axis cross-section of the heart rotates in relation to the position of the papillary muscles.5) Therefore, limitations exist to its use in the observation and quantitative evaluation of twisting.
In 2005, Notomi et al.6) calculated the rotation of the left ventricle from the revolving speed of the myocardial tissue measured using tissue Doppler imaging from the short-axis view. They reported that twisting can be measured from the difference in the magnitude of rotation of the apical and basal short-axis cross-sections, and that twisting measured using this technique agrees well with that determined by MRI tagging technique. However, tissue Doppler imaging has not become widely used for this research, as it is technically demanding to assess rotation. Later, the same investigators showed that rotation and twisting of the ventricle could also be measured with a high degree of accuracy using 2-dimensional speckle tracking echocardiography.7) It has subsequently become possible to measure twisting easily at the bedside, meaning that analysis of twisting motion has attracted considerable attention from echocardiographers.
There are several words relating to wringing such as rotation, twist, torsion, etc. "Rotation" is rotatory movement about the center of the mass in the left ventricular short-axis image.8) Looking from the apex, counterclockwise rotation is expressed with positive values and clockwise rotation with negative values, generally in units of degrees. In the normal heart, the base rotates clockwise during systole and the apex rotates counterclockwise, producing a wringing motion. The difference in turning angle between the base and apex is called the "net twist angle" or "net torsion angle", expressed in degrees. "Torsion" and "twist" are often used interchangeably. While twist is sometimes used simply to mean wringing, torsion is more accurately defined as the base-to-apex gradient in rotation angle along the long-axis of the left ventricle, expressed in degrees per centimeter. Some investigators express torsion as the axial gradient in rotation angle multiplied by the average of the outer radii of apical and basal planes. Properly speaking, this definition would be appropriate for comparing the wringing motion of hearts of different sizes.9) However, because left ventricular long-axis diameter and short-axis diameter change dramatically during a cardiac cycle, this normalization can be used for comparison of only the peak magnitude of torsion. For the sake of simplicity, here I use torsion to mean net twist angle.
Why does the left ventricle rotate, and furthermore, why do the apex and base rotate in opposite directions? To answer these questions, we need to understand the orientations of the myocardial fibers. In the left ventricle, fibers in the subepicardium run in a left-handed direction, fibers in the mid layer run circumferentially, and fibers in the subendocardium run in a right-handed direction (Fig. 1). These myocardial fibers are connected to each other, with a smooth transition from subendcardium to mid layer, and then to subepicardium, about the long-axis. Contraction of these three layers of myocardial fibers causes not only longitudinal, circumferential, and radial movements of the heart, but also contortion of the myocardium.
Myocardial fibers on the subepicardial side run in a left-handed direction, and contraction of these fibers will cause the base to rotate in a clockwise direction and the apex to rotate in a counterclockwise direction. Myocardial fibers on the subendocardial side run in a right-handed direction, and contraction of these fibers will cause the base to rotate in a counterclockwise direction and the apex to rotate in a clockwise direction (Fig. 2). This means that rotations caused by the subepicardium and subendocardium are in opposite directions. Why, then, does clockwise rotation of the base and counterclockwise rotation of the apex become significant? In other words, why is it the rotation of the subepicardial side that becomes significant? This is explained by the difference in the radius of rotation of the subepicardium and subendocardium (Fig. 3). The radius of rotation of the subepicardium is greater than that of the subendocardium. The subepicardium consequently provides greater torque than the subendocardium, as a result of which the rotation of the subepicardium is significantly expressed.
The degree of shortening of myocardial fibers is of the order of 15-20% at most. If ejection was simply the result of contraction of myocardial fibers, the ejection fraction would be 15-20%, whereas the actual ejection fraction of the human heart is 60-70%. This is due to the involvement of twisting. Myocardial fibers are oriented in a spherical fashion, so that when they contract they cause a simultaneous wringing action, resulting in an ejection fraction of 60-70%.10) In addition, after twisting has occurred during systole, untwisting occurs during diastole. Untwisting is known to occur mostly during isovolumic relaxation phase, suggesting that this motion assists left ventricular relaxation (Fig. 4).11) Actually, untwisting is a good index of ventricular relaxation. Dong et al.11) reported a significant negative linear relationship between tau (an index of ventricular relaxation) and recoil rate (untwisting velocity) by MRI and Notomi et al.12) also found a similar relation with echocardiography. They observed it in dogs intervened by dobutamine, esmolol and pacing.
Here, I would like to indicate what rotation and torsion are governed by. I believe rotation (twist) to be governed mainly by three factors: 1) the degree of contraction and relaxation of the myocardium; 2) the balance between contraction of the subendocardium and subepicardium; and 3) orientation of the myocardial fibers. I will explain these in turn.
Rotation is caused by myocardial contraction, so the degree of rotation is evidently governed by the degree of myocardial contraction. Rotation is therefore increased by catecholamines. Fig. 5 shows the relationship between dP/dt and net twist degree. In this dog experiment, dP/dt is controlled by intravenous dobutamine. There is a significant linear relationship suggesting a direct relation between myocardial lengthening and twist.
Rotation is enhanced by raising the preload and is reduced by raising afterload. Dong et al.13) reported higher left ventricular end-diastolic volumes produced higher twist when end-systolic volumes were held constant and higher left ventricular end-systolic volumes produced lower twist when end-diastolic volumes were held constant. This can be well understood by thinking of the relation between the cardiac muscle and loading conditions.
As already noted, despite the fact that contractions of the subendocardium and subepicardium produce rotation in opposite directions, the result is that subepicardial contraction becomes significant due to the difference in torque, so that the base rotates in a clockwise direction and the apex in a conterclockwise direction. Then, what would happen in the presence of dysfunction of the subendocardium? Contraction of the subepicardium would probably become relatively more significant, resulting in increased rotation (hyper-rotation) (Fig. 6). On the other hand, what would happen in the case of subepicardial dysfunction? Rotation of the subepicardium would probably decrease, resulting in hypo-rotation of the ventricle.
Subendocardial dysfunction is well known to appear with myocardial ischemia, hypertension, and many other diseases. Hyper-rotation found in patients suspected of having any of these diseases may indicate subendocardial dysfunction. In other words, measurement of rotation could possibly lead to early detection of such disease.
Diastolic heart failure (heart failure with preserved ejection fraction) has been increasing in recent years.14) Park et al.15) measured rotation and twist in cases of diastolic heart failure, and compared the results with normal subjects. Rotation and twist both showed higher values in the abnormal relaxation (grade 1) group than in the normal group, and values showed a progressive decrease as the degree of diastolic dysfunction advanced to pseudonormalization (grade 2) and restrictive pattern (grade 3) (Fig. 7). Hyper-rotation in the group with abnormal relaxation, which is early-stage diastolic dysfunction, was probably a manifestation of subendocardial dysfunction. Rotation or twist greater than normal values, even with normal ejection fraction, should probably be regarded as an initial stage of diastolic dysfunction. Wang et al.16)17) showed that twisting and untwisting do not decrease with diastolic dysfunction. They measured twisting and untwisting in subjects with contraction disorder, diastolic disorder with preserved ejection fraction, and normal hearts, and found that while values for both twisting and untwisting were low in cases of contraction disorder, these values were not significantly different from normal subjects in diastolic dysfunction cases. Thus, untwisting is not impaired in diastolic dysfunction, at least in its early stage. This means untwisting does not reflect ventricular relaxation. This may make readers confusing because I wrote "untwisting is a good index of ventricular relaxation" above. I think this contradiction may be explained by the difference of disease. Dong et al.11) and Notomi et al.12) observed significant relationship between tau and untwisting velocity in dogs with systolic dysfunction that was created by esmolol. While Park et al.15) and Wang et al.16)17) observed preserved untwisting velocity in patients with diastolic dysfunction with preserved ejection fraction. We probably have to treat differently patients with systolic dysfunction and those with diastolic dysfunction when we try to evaluate diastolic function from untwisting velocity.
Rotation and twisting are known to increase with advancing age,18)19) and this can be explained by the subendocardial dysfunction that is believed to occur with age. In other words, aged people are more or less suffered from diastolic heart failure. Despite of muscular disease, twisting is relatively preserved in hypertrophic cardiomyopathy.20) This seems to be due to reduced subendocardial function resulting from dysfunction of the subendocardial microcirculation. If interstitial fibrosis advances so that there is dysfunction across the full thickness of the myocardium in hypertrophic cardiomyopathy, then rotation will, of course, be reduced.
In some cardiac diseases such as sarcoidosis, muscular dystrophy and myocarditis, subepicardial dysfunction can occur earlier then subendocardial dysfunction. It would be interesting to investigate if there is hypo-rotation with preserved ejection fraction in the early stage of such diseases.
The subendocardial fibers run at an angle of approximately 60° to the long-axis, and the subepicardial fibers run at an angle of approximately 60° in the opposite direction. If the cardiac chambers become rounded as a result of heart failure, the angle becomes greater, resulting that the myocardial fibers run in a more transverse fashion. Rotation and twist are known to be reduced when this occurs. Fig. 8 shows the relationship between left ventricular shape and twist angle.21) Here, the sphericity index is defined as the ratio of the long-axis diameter and the short-axis diameter in diastole. The smaller the sphericity index (i.e., the more spherical the left ventricle becomes), the less the twisting. A parabolic relationship exists between left ventricular shape and twisting, so that twisting is also reduced if the left ventricle becomes overly elliptical. Left ventricular shape is closely associated with the orientation of fibers. When you see the globular left ventricle, its twist would be small. Because the globular left ventricle often accompanies with systolic dysfunction, the reduced twisting may be also due to myocardial dysfunction.
Tanaka et al.22) found rotation and twist decreased in patients with congenital pericardial defect without changes in regional myocardial strains, and they concluded the pericardium played an important role in twist. In fact, this decrease in rotation and twist could probably be explained by left ventricular shape in addition to the role of pericardium. In animal studies, Chang et al.23) measured twist angle before and after incision of the pericardium, and also after the pericardium was resutured. They found that twist was reduced as a result of pericardiotomy and increased again as a result of resuturing of the pericardium. This was attributed to changes in left ventricular shape that occurred as a result of pericardiotomy.
Rotation and twist are concepts that first became familiar to echocardiographers with the appearance of speckle tracking echocardiography. In what ways this knowledge will be of use in clinical settings remains unclear. In addition, we feel there are several limitations in measuring rotation and twist in routine clinical practice. For example, there may be an intervender variability of speckle tracking measurements. Three-dimensional speckle tracking echocardiography, not two-dimensional speckle tracking echocardiography, should be used to avoid the effect of the through plane motion. Nonetheless, as previously stated, there can be no doubt that measuring twist will further our understanding of cardiac mechanics. Also, identification of hyper-rotation probably implies subendocardial dysfunction that is caused by various reasons. I await the day when rotation and twist are recognized as new evaluations of cardiac function.
Figures and Tables
Fig. 1
Myocardial fiber orientation and direction of rotation. Myocardial fibers in the subepicardium helically run in a left-handed direction, fibers in the mid layer run circumferentially, and fibers in the subendocardium helically run in a right-handed direction.
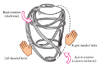
Fig. 2
Myocardial contraction and rotation. When myocardial fibers on the subepicardial side contract, clockwise rotational torque is produced at the base and counterclockwise rotational torque at the apex. When myocardial fibers on the subendocardial side contract, counterclockwise rotational torque is produced at the base and clockwise rotational torque at the apex.

Fig. 3
Opposite rotation at the base and apex. Subepicardial radius is larger than subendocardial radius (r2 > r1). Therefore, subepicardial rotational torque is larger than subendocardial rotational torque (R2 > R1).

Fig. 4
Tagged magnetic resonance imaging of a canine heart. Arrows at the apex mark the initial positions of two tags. By the end of isovolumic relaxation, tags have recoiled almost completely to their starting position indicating that recoil is largely an isovolumic event.11)

Fig. 6
Hyper-rotation in the presence of subendocardial dysfunction. Apical rotation is shown as in Fig. 3. When there is subendocardial dysfunction, RT1' becomes smaller than RT1. Then, because of RT2 >> RT1', hyper-rotation is produced.

Fig. 7
Assessment of diastolic dysfunction based on mitral inflow, mitral annular velocity and left ventricular rotation and torsion. E: peak velocity of the early diastolic filling wave, A: peak velocity of the late diastolic filling wave, E': peak early diastolic annular velocity, A': peak late diastolic annular velocity.15)

Fig. 8
Relationship between left ventricular shape and torsion. Left ventricular shape is expressed by the sphericity index that is the ratio of the long-axis diameter and the short-axis diameter in end-diastole (left). A parabolic relationship is found between the sphericity index and the torsion (twist angle) (right).20)

References
1. Hansen DE, Daughters GT 2nd, Alderman EL, Ingels NB Jr, Miller DC. Torsional deformation of the left ventricular midwall in human hearts with intramyocardial markers: regional heterogeneity and sensitivity to the inotropic effects of abrupt rate changes. Circ Res. 1988. 62:941–952.

2. Gorman JH 3rd, Gupta KB, Streicher JT, Gorman RC, Jackson BM, Ratcliffe MB, Bogen DK, Edmunds LH Jr. Dynamic three-dimensional imaging of the mitral valve and left ventricle by rapid sonomicrometry array localization. J Thorac Cardiovasc Surg. 1996. 112:712–726.

3. Buchalter MB, Weiss JL, Rogers WJ, Zerhouni EA, Weisfeldt ML, Beyar R, Shapiro EP. Noninvasive quantification of left ventricular rotational deformation in normal humans using magnetic resonance imaging myocardial tagging. Circulation. 1990. 81:1236–1244.

4. Buchalter MB, Rademakers FE, Weiss JL, Rogers WJ, Weisfeldt ML, Shapiro EP. Rotational deformation of the canine left ventricle measured by magnetic resonance tagging: effects of catecholamines, ischaemia, and pacing. Cardiovasc Res. 1994. 28:629–635.

5. Mirro MJ, Rogers EW, Weyman AE, Feigenbaum H. Angular displacement of the papillary muscles during the cardiac cycle. Circulation. 1979. 60:327–333.

6. Notomi Y, Setser RM, Shiota T, Martin-Miklovic MG, Weaver JA, Popovic ZB, Yamada H, Greenberg NL, White RD, Thomas JD. Assessment of left ventricular torsional deformation by Doppler tissue imaging: validation study with tagged magnetic resonance imaging. Circulation. 2005. 111:1141–1147.

7. Notomi Y, Lysyansky P, Setser RM, Shiota T, Popovic ZB, Martin-Miklovic MG, Weaver JA, Oryszak SJ, Greenberg NL, White RD, Thomas JD. Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging. J Am Coll Cardiol. 2005. 45:2034–2041.

8. Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging. 2008. 1:366–376.
9. Henson RE, Song SK, Pastorek JS, Ackerman JJ, Lorenz CH. Left ventricular torsion is equal in mice and humans. Am J Physiol Heart Circ Physiol. 2000. 278:H1117–H1123.

10. Buckberg GD. Basic science review: the helix and the heart. J Thorac Cardiovasc Surg. 2002. 124:863–883.

11. Dong SJ, Hees PS, Siu CO, Weiss JL, Shapiro EP. MRI assessment of LV relaxation by untwisting rate: a new isovolumic phase measure of τ. Am J Physiol Heart Circ Physiol. 2001. 281:H2002–H2009.

12. Notomi Y, Popovic ZB, Yamada H, Wallick DW, Martin MG, Oryszak SJ, Shiota T, Greenberg NL, Thomas JD. Ventricular untwisting: a temporal link between left ventricular relaxation and suction. Am J Physiol Heart Circ Physiol. 2008. 294:H505–H513.

13. Dong SJ, Hees PS, Huang WM, Buffer SA Jr, Weiss JL, Shapiro EP. Independent effects of preload, afterload, and contractility on left ventricular torsion. Am J Physiol. 1999. 277:H1053–H1060.
14. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006. 355:251–259.

15. Park SJ, Miyazaki C, Bruce CJ, Ommen S, Miller FA, Oh JK. Left ventricular torsion by two-dimensional speckle tracking echocardiography in patients with diastolic dysfunction and normal ejection fraction. J Am Soc Echocardiogr. 2008. 21:1129–1137.

16. Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Left ventricular untwisting rate by speckle tracking echocardiography. Circulation. 2007. 116:2580–2586.

17. Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J. 2008. 29:1283–1289.

18. Notomi Y, Srinath G, Shiota T, Martin-Miklovic MG, Beachler L, Howell K, Oryszak SJ, Deserranno DG, Freed AD, Greenberg NL, Younoszai A, Thomas JD. Maturational and adaptive modulation of left ventricular torsional biomechanics: Doppler tissue imaging observation from infancy to adulthood. Circulation. 2006. 113:2534–2541.

19. Takeuchi M, Nakai H, Kokumai M, Nishikage T, Otani S, Lang RM. Age-related changes in left ventricular twist assessed by two-dimensional speckle-tracking imaging. J Am Soc Echocardiogr. 2006. 19:1077–1084.

20. Young AA, Kramer CM, Ferrari VA, Axel L, Reichek N. Three-dimensional left ventricular deformation in hypertrophic cardiomyopathy. Circulation. 1994. 90:854–867.

21. van Dalen BM, Kauer F, Vletter WB, Soliman OI, van der Zwaan HB, Ten Cate FJ, Geleijnse ML. Influence of cardiac shape on left ventricular twist. J Appl Physiol. 2010. 108:146–151.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download