Abstract
Background
The aim of this study was to assess the feasibility of targeted ultrasound imaging on apoptosis with annexin A5 microbubbles (A5MB) in acute doxorubicin-induced cardiotoxicity.
Methods
Avidinated and octafluoropropan-filled phospholipid microbubbles were conjugated with biotinylated annexin A5. To confirm the specific binding of A5MB, flow cytometry was performed with hydrogen peroxide induced apoptosis in rat aorta smooth muscle cells incubated with fluorescein-5-isothiocyanate (FITC) labeled annexin A5 and A5MB. Adult male rats were injected intraperitoneally with 5 mg/kg doxorubicin weekly for 3 weeks (n = 5). Control rats were injected with normal saline (n = 5). At 24 hours after the final treatment, triggering imaging was performed 15 min after an intravenous bolus injection of A5MB for washout of freely circulating microbubbles. After echocardiography, the heart was isolated for histological detection of apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay.
Results
In the in vitro tests, fluorescence intensity was low for healthy cells and high for apoptotic cells when incubated with FITC-labeled annexin A5 and A5MB. Rats treated with doxorubicin showed significant contrast opacification of the myocardium on contrast echocardiography using A5MB. However, no opacification was observed in control rats. Apoptosis was confirmed by TUNEL assay in doxorubicin treated rats.
Doxorubicin is a widely-used anticancer agent and the major limitation of its use is dose-related cardiotoxicity.1) At present, doxorubicin cardiotoxicity is routinely screened noninvasively by measurement of the left ventricular (LV) ejection fraction (EF), but abnormal observations can be made only when cardiac damage already has reached significant proportions.2)
Recently, myocardial apoptosis was suggested as a common mechanism of acute and chronic myocyte loss.3-5) In the pathophysiology of cardiovascular disease, programmed cell death of cardiomyocytes has been suggested to be an important contributor because apoptotic cardiomyocytes have been identified during hypoxia, ischemia, cardiac overload, acute myocardial infarction, end-stage heart failure in vivo, and anthracycline use.6)7) Doxorubicin induces apoptosis in several cell lines and, in a rat model, the kidneys, intestines, and cardiomyocytes.7-9) Therefore, the detection of apoptosis could be an opportunity for the noninvasive exploration of early cardiomyopathy.
The detection methods used in most studies evaluating apoptosis of the heart are based on the occurrence of DNA fragmentation, such as the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay and DNA laddering. However, in vivo detection of cell death is not possible with TUNEL or DNA gel electrophoresis.10)
One of the earliest events after triggering cell death is the externalization of phosphatidylserine (PS) to the outer leaflet of the plasma membrane of the cell. Detection of PS exposure can be easily achieved by the phospholipid binding protein annexin A5. In a number of in vitro and in vivo studies, annexin A5 has been demonstrated to be a specific marker for the early and late stages of cells undergoing programmed cell death and that the protein is also suitable for the in situ detection of cell death. Therefore, labeled annexin A5 provides a useful tool for in situ detection of cell death in vivo and also, at least potentially, in clinical settings.11)
Imaging of cellular and molecular events with contrast-enhanced ultrasound has recently been achieved with the use of novel targeted microbubble contrast agents that are retained within diseased organs.12) Unlike inert microbubble blood tracers, targeted microbubbles were designed to adhere to specific endothelial surface epitopes to allow ultrasonic detection of these epitopes.13) The relative advantage of using ultrasound is that it is well-balanced in terms of sensitivity and spatial resolution. In comparison to radionuclide imaging, ultrasound is slightly less sensitive, mostly as a result of the influence of background tissue signal, but has superior spatial resolution. Other potential advantages of ultrasound include its low cost, high temporal resolution, and rapid data acquisition.14)
The aim of this study was to assess the feasibility of targeted ultrasound imaging of apoptosis with microbubbles conjugated with annexin A5 (A5MB) in acute doxorubicin-induced cardiotoxicity models.
Biotinylated microbubbles with lipid shells were prepared by sonication (35 W, 4 minutes) of octafluoropropan gas with aqueous dispersion of 5 mg/mL 1,2-distearoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids, Inc., Alabaster, AL, USA), 5 mg/mL polyethylenglycol distearate (Sigma-Aldrich, St. Louis, MO, USA), and 2.5 mg/mL 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[biotinyl (polyethyleneglycol) 2000] (Avanti Polar Lipids, Inc.) in normal saline. After sonication, the microbubbles were placed in a tube and centrifuged for 3 minutes at 2,000 rpm. The bottom saline was drained and 5 mL of saline was added to the foam and the centrifuge was washed 2 times. Prepared microbubbles were combined with NeutrAvidin (Pierce Biotechnology, Inc., Rockford, IL, USA) for 30 min and washed with saline. Then the microbubbles were combined with biotinylated annexin A5 for 30 min and washed 2 times. Human annexin A5 was produced by expression in Escherichia coli. fluorescein-5-isothiocyanate (FITC)-labeled A5MB for flow cytometry were prepared using FITC-labeled NeutrAvidin.
Specific binding of A5MB to apoptotic cells was confirmed by flow cytometry. Rat aorta smooth muscle cells (SMC) were cultured over 70% confluency in Dulbecco's modified Eagle's medium (GIBCO BRL, Rockville, MD, USA) with 10% fetal bovine serum and treated with hydrogen peroxide 100 µM. Three hours after the treatment, the cells were collected and washed with phosphate-buffered saline (PBS) and then resuspended in a binding solution of 500 µL (2% BSA, 10 mM HEPES, 150 mM NaCl, 2.5 mM CaCl2, 1 mM MgCl2) and blocked for 1 hour at room temperature. FITC-labeled annexin A5 and FITC-labeled A5MB were added to cell suspensions. The reaction was incubated in the dark for 20 minutes at room temperature and the cells were washed 2 times with PBS. Samples were placed on a tube and immediately analyzed on a FACS Calibur (Becton Dickinson, Franklin Lakes, NJ, USA) to generate histograms of green fluorescence intensity.
Adult male Sprague-Dawley rats weighing 247 ± 6 g were purchased from Harlan and maintained under standard conditions at an animal care facility. The rats had free access to standard rodent chow and water. After rats received subcutaneous buprenorphine (0.05 mg/kg; Hanlim Pharm., Seoul, Korea) to provide analgesia, doxorubicin (Dong-A Pharm., Seoul, Korea) 5 mg/kg was injected intraperitoneally. This treatment was repeated weekly for 3 weeks, resulting in a total cumulative dose of 15 mg/kg per animal (n = 5). Control rats were injected with the same volume of buprenorphine and physiological saline instead of doxorubicin (n = 5). All the experiments were performed according to the "Revised Guide for the Care and Use of Laboratory Animals Available".15)
Rats were sedated with zoletil (50 mg/kg) and xylazine (5 mg/kg), which was administered intraperitoneally. Once sedated, the femoral vein was cannulated for microbubble administration.
Imaging was performed at 14 MHz with a linear-array transducer interfaced with an ultrasound system (Vivid 7, GE Vingmed Ultrasound, Horten, Norway). Images were acquired in a parasternal short axis view with the transducer fixed in position with a free-standing clamp. Before microbubble injection, baseline images were acquired. Gain settings, depth, and focus were initially optimized and maintained throughout the experiment. Ultrasound transmission was then suspended, and 4 × 106 A5MB were injected as an intravenous bolus.
Preliminary studies demonstrated that a bolus of 4 × 106 microbubbles resulted in visually strong, reproducible opacification of rat myocardium and the myocardial contrast was no longer detectable by 15 minutes after injection. Based on these observations, myocardial backscatter at 15 minutes should derive predominantly from adherent microbubbles and less so from the few remaining circulating microbubbles.
Immediately after contrast injection, a very high concentration of freely circulating microbubbles in the blood pool was expected. Therefore, imaging was not resumed until 15 min after injection for retention of microbubbles in apoptotic tissue and clearance of freely circulating microbubbles in the blood pool. Intermittent electrocardiography-triggering imaging (mechanical index of 0.8) was then initiated at a pulse interval of 1 cardiac cycle for several frames. Contrast opacification of myocardium in the 1st frame was considered as the signal coming from the adhered microbubbles. Therefore, contrast enhancement in the myocardium in the 1st frame and its disappearance within the subsequent frames were considered as positive results. Images were recorded digitally and analyzed offline.
The TUNEL assay was performed according to the manufacturer's instructions (Chemicon, Temecula, CA, USA). In brief, the excised heart tissues were fixed in 3.7% buffered formaldehyde and embedded in paraffin. Five µm-thick tissue sections were deparaffinized, rehydrated, and rinsed with PBS. A positive control sample was prepared by treating normal heart tissue with DNase I (10 U/mL, 10 min at room temperature). Sections were pretreated with 3.0% H2O2, subjected to the reaction with TdT enzymes for 37℃ for 1 hour and incubated in digoxigenin-conjugated nucleotide substrate at 37℃ for 30 min. Nuclei exhibiting DNA fragmentation were performed by 3,3-diamino benzidine for 5 min. Apoptotic cardiomyocytes nuclei were stained dark brown. Lastly, sections were counterstained with methyl green and coverslipped. The sections were observed by light microscopy.
At the beginning of the experiment and before administering any treatment, baseline echocardiography was performed in both groups to measure LV dimensions and LV EF. Then, doxorubicin or saline treatment was begun and repeated weekly for 3 weeks according to the experiment group described above. At 24 hours after the final treatment, LV performance was examined by conventional echocardiography and targeted ultrasound imaging using A5MB was obtained as described above. Immediately after echocardiography, the rats were sacrificed and the entire heart was removed and processed for histological analysis.
The histograms of fluorescence intensity obtained by flow cytometry represent specific bindings of FITC labeled annexin-5-microbubbles to apoptotic SMC (Fig. 1). Compared to healthy cells, apoptotic cells incubated with FITC-labeled A5MB were characterized by a higher percentage of positive fluorescence staining (3.7% vs. 91.4%). Fluorescence intensity was low for healthy cells and increased when apoptotic cells were incubated with FITC-labeled annexin A5 and FITC-labeled A5MB. These results indicated that the A5MB bind specifically to apoptotic cells.
The body weight of control rats was significantly increased at the end of the experiment and LV mass was slightly increased during the experiment period in both groups. However, no significant change in LV dimensions and LV EF was observed during the experiment in either group (Table 1). There was also no significant difference in body weight and LV performance between the two groups.
Fig. 2 demonstrated the results of contrast echocardiography using A5MB. In rats treated with doxorubicin, the 1st frame showed significant contrast opacification of myocardium; the signal coming from the adhered microbubbles (Fig. 2A). These retained microbubbles were destroyed during subsequent high mechanical index imaging. Therefore, no opacification was observed in the 10th frame (Fig. 2B). In control rats, unbound microbubbles dissipated during the waiting period and no contrast enhancement was observed in the 1st (Fig. 2C) or the 10th frames (Fig. 2D).
The result of the TUNEL assay revealed increased apoptosis frequencies for both the cardiomyocytes and endothelial cells of doxorubicin-treated hearts compared to control hearts (Fig. 3). TUNEL-positive cardiomyocytes and endothelial cells were significantly higher in the doxorubicin-treated group than in the control group, which indicated that doxorubicin induced apoptosis of cardiomyocyte and endothelial cells (Fig. 4).
The main finding of this study is that targeted ultrasound imaging using A5MB is capable of identifying apoptosis in acute doxorubicin induced cardiomyopathy. This study is the first description of an ultrasound approach for detecting apoptosis using a targeted contrast agent and has major implications for noninvasive detection of early apoptosis in doxorubicin induced cardiomyopathy. In addition, it is encouraging that apoptosis detection by ultrasound is possible in this study before definite LV systolic dysfunction occurs.
Molecular imaging is a rapidly evolving discipline with the goal of developing tools to display and quantify molecular and cellular targets in vivo.16) Although techniques for molecular imaging have been developed for essentially every form of medical imaging, there are significant differences that influence the choice of imaging methods in both research and clinical settings. For the imaging of apoptosis, recombinant human annexin A5 has been radiolabeled with 99mTc, and the feasibility of imaging apoptosis has been demonstrated both in experimental and clinical cardiovascular disease states.17-19) Bennink et al.20) recently showed that acute doxorubicin induced cardiomyopathy based on early apoptosis can be imaged with 99mTc-annexin A5 scintigraphy in the murine model.
In this study, we used microbubbles conjugated with annexin A5 for targeted ultrasound imaging of apoptosis. In comparison to radionuclide imaging, the high temporal resolution, availability, rapid execution of imaging protocols, and relatively low cost are features of targeted molecular imaging with ultrasound that make this technique attractive.12) As microbubbles are pure intravascular tracers, molecular imaging with contrast-enhanced ultrasound has focused on diseases such as inflammation, thrombus formation, and angiogenesis, which are mediated by pathophysiologic events that occur within the vasculature.14) In doxorubicin-induced cardiotoxicity, both cardiomyocyte and endothelial cell death can occur via apoptosis.21) Therefore, in this study, retained microbubbles that produced contrast enhancements were thought to be adhered to the apoptotic endothelium, not to the apoptotic cardiomyocyte. However, we could not entirely exclude the possibility of direct attachment of A5MB to apoptotic cardiomyocytes by extravasation through the disrupted microvessel. In this study, the direct visualization of A5MB binding to apoptotic cell could not be performed in in vitro tests nor in in vivo experiments.
The results of this study indicate that site-targeted ultrasound has the potential for non-invasive investigation of early apoptosis preceding deterioration of LV systolic function in doxorubicin induced cardiomyopathy. However, a destructive imaging protocol that includes a 15-minute delay for wash-out may be difficult to apply clinically in the future, because precisely locating the heart before the first destructive pulse may be challenging. In addition, contrast opacification was assessed by visual estimation rather than by a quantitative method in this study, and a newer quantification method may improve the reproducibility and practicability of targeted ultrasound imaging.
In conclusion, acute doxorubicin-induced cardiomyopathy based on early apoptosis can be assessed and imaged with targeted ultrasound imaging using microbubbles conjugated with annexin A5 in rats. This investigation model may lead to interesting applications during in vivo evaluations of cardiomyocyte apoptosis in cardiomyopathy.
Figures and Tables
 | Fig. 1Specific binding of FITC-labeled annexin A5 to apoptosis cells. Healthy cells (A). Healthy cells incubated with FITC-labeled annexin A5 (B). Healthy cells incubated with microbubbles conjugated with FITC-labeled annexin A5 (C). Apoptotic cells incubated with FITC-labeled annexin A5 (D). Apoptotic cells incubated with microbubbles conjugated with FITC-labeled annexin A5 (E). FITC: fluorescein-5-isothiocyanate. |
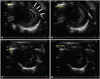 | Fig. 2Targeted ultrasound imaging with annexin A5 conjugated microbubbles in doxorubicin-treated rats (A and B) and control rats (C and D). Significant contrast opacification of myocardium (arrows) was observed in the 1st frame (A). Initial contrast enhancement was dissipated during subsequent high mechanical index imaging in the 10th frame (B). No contrast opacification was observed in the myocardium in the 1st frame (C). No contrast opacification was observed in the myocardium in the 10th frame (D). |
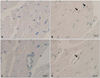 | Fig. 3In situ labeling of DNA fragmentation in rat myocardium from TUNEL assay. Fragmented DNA is labeled in brown, and all nuclei are counterstained with methyl-green. Myocardial sections from rats in the control group reveal no evidence of DNA fragmentation in cardiomyocytes (A) or endothelial cells (C). A heterogenous pattern is revealed for the isolated apoptotic nuclei in the myocardium of doxorubicin-treated rats, as indicated by arrows, for both cardiomyocytes (B) and endothelial cells (D). TUNEL: terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling. |
 | Fig. 4The quantitative results of positive TUNEL-staining cardiomyocytes and endothelial cells. The ratio of TUNEL-positive cardiomyocytes (A) and endothelial cells (B) are significantly higher in the doxorubicin-treated group (Doxo) than in the control group (Con). *p < 0.01 vs. control. TUNEL: terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling. |
Acknowledgements
This work was supported by grants from the Korean Society of Echocardiography (Industrial-Educational Cooperation, 2005).
References
1. Bristow MR, Billingham ME, Mason JW, Daniels JR. Clinical spectrum of anthracycline antibiotic cardiotoxicity. Cancer Treat Rep. 1978. 62:873–879.
2. Nousiainen T, Jantunen E, Vanninen E, Hartikainen J. Early decline in left ventricular ejection fraction predicts doxorubicin cardiotoxicity in lymphoma patients. Br J Cancer. 2002. 86:1697–1700.

3. Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation. 1997. 95:320–323.

4. Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997. 336:1131–1141.

5. Saraste A, Pulkki K, Kallajoki M, Heikkilä P, Laine P, Mattila S, Nieminen MS, Parvinen M, Voipio-Pulkki LM. Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur J Clin Invest. 1999. 29:380–386.

6. Nakamura T, Ueda Y, Juan Y, Katsuda S, Takahashi H, Koh E. Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: In vivo study. Circulation. 2000. 102:572–578.

7. Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000. 60:1789–1792.
8. Ling YH, Priebe W, Perez-Soler R. Apoptosis induced by anthracycline antibiotics in P388 parent and multidrug-resistant cells. Cancer Res. 1993. 53:1845–1852.
9. Zhang J, Clark JR Jr, Herman EH, Ferrans VJ. Doxorubicin-induced apoptosis in spontaneously hypertensive rats: differential effects in heart, kidney and intestine, and inhibition by ICRF-187. J Mol Cell Cardiol. 1996. 28:1931–1943.

10. van Heerde WL, Robert-Offerman S, Dumont E, Hofstra L, Doevendans PA, Smits JF, Daemen MJ, Reutelingsperger CP. Markers of apoptosis in cardiovascular tissues: focus on Annexin V. Cardiovasc Res. 2000. 45:549–559.

11. van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998. 31:1–9.

12. Kaufmann BA, Lindner JR. Molecular imaging with targeted contrast ultrasound. Curr Opin Biotechnol. 2007. 18:11–16.

13. Klibanov AL. Targeted delivery of gas-filled microspheres, contrast agents for ultrasound imaging. Adv Drug Deliv Rev. 1999. 37:139–157.

14. Lindner JR. Molecular imaging with contrast ultrasound and targeted microbubbles. J Nucl Cardiol. 2004. 11:215–221.

15. Bayne K. American Physiological Society. Revised Guide for the Care and Use of Laboratory Animals available. Physiologist. 1996. 39:199208–211.
16. Jaffer FA, Weissleder R. Seeing within: molecular imaging of the cardiovascular system. Circ Res. 2004. 94:433–445.
17. Narula J, Acio ER, Narula N, Samuels LE, Fyfe B, Wood D, Fitzpatrick JM, Raghunath PN, Tomaszewski JE, Kelly C, Steinmetz N, Green A, Tait JF, Leppo J, Blankenberg FG, Jain D, Strauss HW. Annexin-V imaging for noninvasive detection of cardiac allograft rejection. Nat Med. 2001. 7:1347–1352.

18. Hofstra L, Liem IH, Dumont EA, Boersma HH, van Heerde WL, Doevendans PA, De Muinck E, Wellens HJ, Kemerink GJ, Reutelingsperger CP, Heidendal GA. Visualisation of cell death in vivo in patients with acute myocardial infarction. Lancet. 2000. 356:209–212.

19. Dumont EA, Reutelingsperger CP, Smits JF, Daemen MJ, Doevendans PA, Wellens HJ, Hofstra L. Real-time imaging of apoptotic cell-membrane changes at the single-cell level in the beating murine heart. Nat Med. 2001. 7:1352–1355.

20. Bennink RJ, van den Hoff MJ, van Hemert FJ, de Bruin KM, Spijkerboer AL, Vanderheyden JL, Steinmetz N, van Eck-Smit BL. Annexin V imaging of acute doxorubicin cardiotoxicity (apoptosis) in rats. J Nucl Med. 2004. 45:842–848.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download