Abstract
Subaortic stenosis usually occurs without a previous heart operation, however, it can occur after heart surgery as well, with a condition known as a secondary subaortic stenosis (SSS). SSS has been reported after surgical repair of several congenital heart defects. There are only a few recorded cases of SSS after repair of ventricular septal defect (VSD). Here we report a rare case of SSS that occurred 3 years after surgical repair of subarterial VSD. A follow-up echocardiogram is essential for detecting SSS caused by the newly developed subaortic membrane in patients who had cardiac surgery.
Subaortic stenosis (SAS) is a consequence of left ventricular outflow tract (LVOT) obstruction. Obstruction may be the result of either an actual ridge of fibromuscular origin or a fib-rous tissue that coats and tethers the aortic valve (AV) leaflets.1)2) SAS can be divided into two main categories: discrete type and tunnel type. Discrete type is more common, and is caused by a discrete subvalvular membrane or muscular band.1)
SAS usually occurs without a previous heart operation, but it can also occur after heart surgery, with a condition known as secondary subaortic stenosis (SSS).3) SSS has been reported after surgical repair of several congenital heart defects, with or without an initial LVOT obstruction, and occurs most often after repair of coarctation of the aorta, Fontan procedure, or Rastelli operation.4) Only few cases of SSS after repair of ventricular septal defect (VSD) have been reported.5)
We report on a rare case of SSS that occurred 3 years after surgical repair of subarterial VSD.
A 3-year-old boy visited our outpatient department due to cardiac murmur. The patient was transferred to our hospital 3 days after a premature birth with esophageal atresia, and a cardiac murmur, and underwent an esophago-esophagostomy at 1 month old. Two-dimensional echocardiography showed a large subarterial VSD (Fig. 1). The patient subsequently underwent a patch closure of subarterial VSD at 3 months old. The VSD shunt disappeared after surgery (Fig. 2). During follow-up, no specific problems were noted on physical examination or radiologic findings.
There was grade 2/6 systolic murmur at the left sternal border on physical examination. He showed no subjective symptoms including exercise intolerance. Chest X-ray showed no cardiomegaly. Electrocardiography revealed left ventricular hypertrophy. Two-dimensional echocardiography showed mild SAS due to discrete subaortic membrane (Fig. 3). Peak pressure gradient was estimated at 24 mmHg (Fig. 4A). M-mode echocardiography showed a mid-systolic partial closure and fluttering right coronary cusp of AV (Fig. 4B).
We recommended resection of the subaortic membrane when LVOT pressure gradient or aortic insufficiency (AI) progressed during serial echocardiography.
SAS usually occurs without previous heart operation, but it can be occurred after surgical repair of several congenital heart defects, including univentricular heart requiring the Fontan procedure,6) VSD,5) tetralogy of Fallot,5) double outlet right ventricle,4)5) Shone's syndrome,7) abnormal ventriculoarterial connections,6) partial and complete atrioventricular septal defect,8) and common atrium, and aorticopulmonary window.3) In one study, postsurgical SAS appeared to be an uncommon complication in patients with VSD and tetralogy of Fallot, with frequency of 3.2% and 2.1% respectively. However, frequency was relatively higher, at 21.4%, in patients with a double outlet right ventricle.5)
Pathophysiological theories, including turbulence theory or geometric theory, for development of SAS without a previous heart operation can be extrapolated to SSS.3) Anatomical elements, such as a defect of the AV, hypoplastic aortic annulus, and subaortic narrowing can cause turbulent flow patterns in the subaortic region, and these may have contributed to development of SSS through stimulation of the endothelium.3)
SSS development could be related to scar formation in the LVOT.3) The initial surgical procedure may contribute in a number of ways to development of SSS. The left ventricle (LV) undergoes geometric change after the Rastelli operation or intraventricular repair. After an operation for isolated VSD or tetralogy of Fallot, LV hypertrophy and extension of the VSD patch into the LVOT can also lead to SSS formation.9) After 2 years of initial surgery, 71% of SSS appeared with mean interval of 4.4 years.3) Whatever the initial heart defect, a young age at the time of initial surgery was a significant risk factor for developing SSS.10)
The majority of SSS patients were asymptomatic. A diagnosis of SSS was suspected on routine follow-up Doppler echocardiography. Two-dimensional echocardiography with color Doppler imaging is the current modality of choice to establish a diagnosis of SSS. It is helpful in defining and localizing SAS, and reveals the extent of LVOT involvement, degree of LV hypertrophy, indices of LV performance, and parameters of diastolic function in the LV. Secondary effects, such as degree of AI, mitral regurgitation, or poststenotic dilatation of the aorta, can also be assessed. M-mode echocardiography provides indirect evidence of SSS by revealing early closure due to the Venturi effect of the jet formed by the SSS and the coarse flutter of the AV leaflets.11)
SAS is a progressive and serious disease causing severe LV hypertrophy and significant AI.12) LV hypertrophy and AI can occur due to AV damage because of the jet from subaortic narrowing, which may also render the AV prone to infective endocarditis.13)14) A significant LV-aortic mean gradient of more than 30 mmHg in children require surgery.15)
This report is a case of SSS that occurred 3 years after patch closure of subarterial VSD. A follow-up echocardiogram is essential for detecting SSS caused by the newly developed subaortic membrane in patients who had cardiac surgery.
Figures and Tables
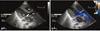
Fig. 1
Color Doppler imaging from parasternal long axis view (A) and parasternal short axis view (B) shows a large subarterial ventricular septal defect (arrows) at initial diagnosis. RV: right ventricle, LV: left ventricle, LA: left atrium, Ao: aorta, PA: pulmonary artery.

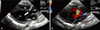
Fig. 2
After patch closure of the subartrial ventricular septal defect, echocardiography shows no discrete subaortic membrane (arrow) (A) and no blood flow disturbance in the left ventricular outflow tract (B). RV: right ventricle, LV: left ventricle, LA: left atrium, Ao: aorta.
References
1. Erentug V, Bozbuga N, Kirali K, Goksedef D, Akinci E, Isik O, Yakut C. Surgical treatment of subaortic obstruction in adolescent and adults: long-term follow-up. J Card Surg. 2005. 20:16–21.

2. Cho YS, Chang KS, Chin YK, Park KH, Youn SJ, Seo JC, Kim GY, Hong SP. Clinical observation on congenital heart disease in adult. J Korean Soc Echocardiogr. 1999. 7:23–31.

3. Kalfa D, Ghez O, Kreitmann B, Metras D. Secondary subaortic stenosis in heart defects without any initial subaortic obstruction: a multifactorial postoperative event. Eur J Cardiothorac Surg. 2007. 32:582–587.

4. Takeuchi K, McGowan FX Jr, Moran AM, Zurakowski D, Mayer JE Jr, Jonas RA, del Nido PJ. Surgical outcome of double-outlet right ventricle with subpulmonary VSD. Ann Thorac Surg. 2001. 71:49–52. discussion 52-3.

5. Cicini MP, Giannico S, Marino B, Iorio FS, Corno A, Marcelletti C. "Acquired" subvalvular aortic stenosis after repair of a ventricular septal defect. Chest. 1992. 101:115–118.

6. Razzouk AJ, Freedom RM, Cohen AJ, Williams WG, Trusler GA, Coles JG, Burrows PE, Rebeyka IM. The recognition, identification of morphologic substrate, and treatment of subaortic stenosis after a Fontan operation. An analysis of twelve patients. J Thorac Cardiovasc Surg. 1992. 104:938–944.

7. Serraf A, Zoghby J, Lacour-Gayet F, Houel R, Belli E, Galletti L, Planche C. Surgical treatment of subaortic stenosis: a seventeen-year experience. J Thorac Cardiovasc Surg. 1999. 117:669–678.

8. Gurbuz AT, Novick WM, Pierce CA, Watson DC. Left ventricular outflow tract obstruction after partial atrioventricular septal defect repair. Ann Thorac Surg. 1999. 68:1723–1726.

9. Lampros TD, Cobanoglu A. Discrete subaortic stenosis: an acquired heart disease. Eur J Cardiothorac Surg. 1998. 14:296–303.

10. Brauner R, Laks H, Drinkwater DC Jr, Shvarts O, Eghbali K, Galindo A. Benefits of early surgical repair in fixed subaortic stenosis. J Am Coll Cardiol. 1997. 30:1835–1842.

11. Kleinert S, Geva T. Echocardiographic morphometry and geometry of the left ventricular outflow tract in fixed subaortic stenosis. J Am Coll Cardiol. 1993. 22:1501–1508.

12. van Son JA, Schaff HV, Danielson GK, Hagler DJ, Puga FJ. Surgical treatment of discrete and tunnel subaortic stenosis. Late survival and risk of reoperation. Circulation. 1993. 88:II159–II169.
13. Cohen L, Bennani R, Hulin S, Malergue MC, Yemets I, Kalangos A, Murrith N, Ouaknine R, Lecompte Y. Mitral valvar anomalies and discrete subaortic stenosis. Cardiol Young. 2002. 12:138–146.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download