Abstract
Left ventricular (LV) pseudoaneurysms rarely occur, but are detected more often with the development of new diagnostic tools. Since LV pseudoaneurysms are life-threatening, early surgical intervention is recommended. This report describes an 87-year-old woman with heart failure and a large LV pseudoaneurysm which progressed from a small LV pseudoaneurysm after an acute myocardial infarction over a 1-year period.
Left ventricular (LV) pseudoaneurysms are rare clinical conditions that could be life-threatening. LV pseudoaneurysms occur when the myocardial rupture is sealed by pericardium and fibrous tissue. Myocardial infarction (MI) is the most common cause of LV pseudoaneurysms, followed by cardiac surgery, trauma, and infection.1) LV pseudoaneurysms are characterized by rapid growth, a narrow neck, and a large external sac.2) Unlike true aneurysms, pseudoaneurysms can rupture and early surgical intervention is recommended. This report describes an elderly woman with heart failure and a large LV pseudoaneurysm which progressed from a small LV pseudoaneurysm after an acute MI over a 1-year period.
An 87-year-old woman visited the emergency department for worsening severe dyspnea and chest discomfort. On admission, the blood pressure was 150/90 mmHg, the respiratory rate was 36 per minute, the pulse rate was 112 per minute, and the peripheral oxygen saturation was 80%. Fine crackles were auscultated in both lower lung fields and an apical grade 2/6 holosystolic murmur was present. A chest radiograph revealed cardiomegaly and pulmonary congestion (Fig. 1A). The electrocardiogram showed voltage criteria consistent with LV hypertrophy, and T wave inversion in leads V5 and V6 was compatible with LV strain (Fig. 1B).
The CK-MB and troponin-I levels were normal (1.87 and 0.022 ng/mL, respectively). The B-type natriuretic peptide was markedly elevated (1,850 pg/mL). Other laboratory parameters were unremarkable.
The patient had a history of an acute MI during a previous admission; the electrocardiogram showed ST segment elevation in leads III and aVF and Q waves in leads II, III, and aVF. Coronary angiography revealed severe coronary artery stenoses (total occlusion of the proximal right coronary artery, total occlusion of the proximal left circumflex artery, and a 40% stenotic lesion in the distal left main artery). Thus, primary percutaneous coronary revascularization of the right coronary artery was performed.
Two-dimensional echocardiography at the previous admission showed decreased LV systolic function (ejection fraction, 40%) and a mild pericardial effusion. The regional wall motion abnormalities with akinetic basal to the mid-inferior and posterolateral walls of the LV were observed. In a color Doppler study, mild mitral regurgitation was noted in systole. The continuity of the myocardium of the mid-posterior wall was disrupted and a small sac (22 × 11 mm) with a narrow neck was seen which was suspected to be a rupture of the free wall with a thrombotic plug (Fig. 2A). A LV pseudoaneurysm was diagnosed and contrast echocardiography was performed to evaluate further blood leakage through the ruptured myocardium and sac. Contrast echocardiography revealed that the pseudoaneurysm on the LV posterior wall was clearly defined and did not communicate with the pericardial space (Fig. 2B). Cardiac magnetic resonance imaging (MRI) also showed a small bulging sac-like lesion with a neck portion in the mid-posterior wall of the LV without definite myocardial tissue (Fig. 3).
The patient and her family declined to undergo surgery for the LV pseudoaneurysm. The patient was discharged after a few days of medical therapy and did not return for follow-up.
During the admission, two-dimensional echocardiography revealed an increase in the size of the LV and decreased LV systolic function (ejection fraction, 30%). A large cavity in the posterior area of the mid-posterior wall of the LV (> 80 × 55 mm) was noted which was diagnosed as a small LV pseudoaneurysm 1 year earlier (Fig. 4A and B). Blood flow across the hole from the LV to the cavity in systole (Fig. 4B) and from the cavity to the LV in diastole was observed by color Doppler study (Fig. 4C). Mild-to-moderate mitral regurgitation in systole and diastole was noted in a color Doppler study (Fig. 4C and D) that was more severe compared with the previous admission. A small LV pseudoaneurysm after an acute MI progressed to a huge pseudoaneurysm with significant mitral regurgitation in 1 year without any treatment. Surgical management was strongly recommended, but she declined again and was discharged when dyspnea had improved with medication for congestive heart failure.
A LV pseudoaneurysm generally occurs after transmural infarction and is characterized by complete rupture of the myocardium with extravasated blood by overlying adherent pericardium. LV pseudoaneurysms have been reported to occur primarily in the posteroinferior wall, and in basal segments rather than in apical segments.1) One suggested explanation for the relative lack of anterior LV pseudoaneurysms is that anterior rupture may more likely result in a hemopericardium and death than a posterior rupture.3) Hospitalized patients are usually in the recumbent position, therefore an inflammatory reaction of the posterior pericardium may result in pericardial adhesions and the formation of a posterior LV pseudoaneurysm rather than cardiac tamponade.1)
The most common clinical presentation of pseudoaneurysms is characterized by congestive heart failure (36%), chest pain (30%), and dyspnea (25%), whereas the incidence of sudden death as a presenting symptom is 3%.4) In our case, the patient presented with atypical chest pain at the time of first admission and congestive heart failure at the time of re-admission. The diagnosis of pseudoaneurysms is complicated because the symptoms of acute pseudoaneurysm are similar to the symptoms of a MI and chronic pseudoaneurysms are similar with symptoms of congestive heart failure. Although the classic sign of a pseudoaneurysm is a new to-and-fro murmur,5) it has been previously reported that the murmur may be indistinguishable from mitral regurgitation (MR)6) or absent in approximately 30% of cases.7)
The early diagnosis of LV pseudoaneurysms is essential to avoid complications and to determine appropriate treatment. Echocardiography is a valuable non-invasive test for diagnosing pseudoaneurysms, which produce a bounded echo-free space with the orifice of a narrow neck communicating with the LV. The maximal diameter of the orifice is smaller than the maximal aneurysmal diameter (< 50%) and abnormal bidirectional blood flow occurs across the orifice.8) The orifice of the pseudoaneurysm is generally smaller than the orifice of a true aneurysm.9) Pseudoaneurysms are more frequently located in the posteroinferior wall than the anterior wall, while true aneurysms are located more frequently in the anterior wall and apex.8) Contrast echocardiography offers enhanced endocardial border delineation of the LV,10) and better visualization of the pseudoaneurysmal border. Contrast echocardiography is helpful to diagnose small leakage from the LV for detection of LV rupture.
Recent reports have indicated that cardiac multi-slice computed tomography is a sensitive technique for detecting LV pseudoaneurysms.11) Moreover, cardiac MRI may represent an effective diagnostic tool as cardiac MRI is able to distinguish among the pericardium, myocardium, and thrombi, and visualize disruption of the epicardial fat layer at the site of a pseudoaneurysm.12) Echocardiography is a valuable and simple method to facilitate the diagnosis and evaluation of pseudoaneurysms. Echocardiography allows a rapid bedside assessment and is easily available in the emergency department.
Surgical resection is considered the treatment of choice for LV pseudoaneurysms because of the risk of rupture. The endocardial patch technique is recommended in the acute phase and for posterior pseudoaneurysms, whereas chronic anterior pseudoaneurysms are closed primarily.2) It is generally accepted that high mortality rates exist for patients with LV pseudoaneurysms who do not undergo surgery. However, one study reported slightly prolonged survival in some patients who were treated conservatively.1)
LV pseudoaneurysms rarely occur, but are observed more often with the development of new diagnostic tools. However, LV pseudoaneurysms are rarely observed to progress after an acute MI in the same patient, as in the case presented herein.
Figures and Tables
Fig. 1
Chest radiograph (A) shows cardiomegaly with a cardiothoracic ratio of 75%, pulmonary congestion, and a tortuous aorta. The electrocardiography (B) shows voltage criteria of LV hypertrophy and T wave inversion in leads V5 and V6, compatible with LV strain. LV: left ventricle.

Fig. 2
During the previous admission, transthoracic two-dimensional echocardiography (A) shows an echo-free space (arrow) with a maximal diameter of 22×11 mm and a neck of 15×17 mm. The myocardium at the neck abruptly stops, and a thrombotic plug is observed. Contrast echocardiography (B) shows better margin of the pseudoaneurysm (arrow) and no clear visualization of dye leakage to the pericardial space. LV: left ventricle.

Fig. 3
Cardiac magnetic resonance imaging during the previous admission shows a focal, bulging, sac-like lesion (arrow) without a definite peripheral wall in the lateral wall at the mid-LV level. LV: left ventricle.
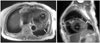
Fig. 4
Transthoracic two-dimensional echocardiography on re-admission shows a large, echo-free space (*) behind the posterior wall, which communicates with the left ventricle through a narrow orifice, and an abrupt interruption of the myocardium at the neck is shown (A and B). The maximal diameter of the cavity is 80×55 mm and that of the orifice is 14×18 mm. A color Doppler study shows the blood fiows across the orifice from the LV to the cavity in systole (C) and from the cavity to the LV in diastole (D). Mild-to-moderate mitral regurgitation is observed in both systole (C) and diastole (D). LV: left ventricle.

References
1. Frances C, Romero A, Grady D. Left Ventricular Pseudoaneurysm. J Am Coll Cardiol. 1998. 32:557–561.

2. Nurozler F, Kutlu T, Küçük G. False aneurysm of the left ventricle following myocardial infarction: an unusual location. Cardiovasc J Afr. 2007. 18:380–382.
3. Rittenhouse EA, Sauvage LR, Mansfield PB, Smith JC, Davis CC, Hall DG. False aneurysm of the left ventricle. Report of four cases and review of surgical management. Ann Surg. 1979. 189:409–415.
4. Contuzzi R, Gatto L, Patti G, Goffredo C, D'Ambrosio A, Covino E, Chello M, Di Sciascio G. Giant left ventricular pseudoaneurysm complicating an acute myocardial infarction in patient with previous cardiac surgery: a case report. J Cardiovasc Med (Hagerstown). 2009. 10:81–84.

5. Turgeman Y, Antonelli D, Rosenfeld T. Intermittent to-and-fro murmur in cardiac pseudoaneurysm: Doppler echocardiographic findings. Int J Cardiol. 1990. 26:376–377.

6. MacNeil DJ, Vieweg WV, Oury JH, Folkerth TL, Hagan AD. Pseudomitral regurgitation due to false aneurysm of the left ventricle treated successfully by surgery. Chest. 1974. 66:724–726.

7. March KL, Sawada SG, Tarver RD, Kesler KA, Armstrong WF. Current concepts of left ventricular pseudoaneurysm: pathophysiology, therapy, and diagnostic imaging methods. Clin Cardiol. 1989. 12:531–540.

8. Brown SL, Gropler RJ, Harris KM. Distinguishing left ventricular aneurysm from pseudoaneurysm. A review of the literature. Chest. 1997. 111:1403–1409.

9. Park JR, Kho JS, Im SI, Park JY, Choi BR, Park SJ, Kwak CH, Hwang JY, Jeon KN. A case of left ventricular pseudoaneurysm extending to lateral side of left atrium after myocardial infarction. J Cardiovasc Ultrasound. 2006. 14:29–32.

10. Pandian NG. Clinical applications of contrast echocardiography. Eur J Echocardiogr. 2004. 5:Suppl 2. S3–S10.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download