Abstract
Carbon monoxide (CO) is one of well known chemical asphyxiants which cause tissue hypoxia with prominent neurologic and cardiovascular injury. Cardiac dysfunction after CO poisoning can be presented as two clinical patterns. One is transient global left ventricular (LV) dysfunction and the other is LV dysfunction with regional wall motion abnormalities. In this case report, we present a case with transient LV systolic dysfunction caused by intentional exposure to CO. After conservative treatment including high concentration of oxygen, the patient recovered completely without any complication.
Carbon monoxide (CO) can cause functional and morphological alternations of the heart mainly due to myocardial hypoxemia and direct action of CO on the heart.1)2) CO has about 250-fold higher affinity for hemoglobin as compared to oxygen and forms carboxyhemoglobin (CO-Hb). In the presence of CO-Hb, a leftward shift of the oxygenated hemoglobin dissociation curve observed and leads to impairment of tissue oxygen delivery and makes cellular hypoxia.1)
CO induced cardiotoxicity has many clinical manifestations including arrhythmias, pulmonary edema and heart failure, and myocardial infarction. Echocardiography is known as the most useful method in the detection the presence of cardiac toxicity and assessment of its severity. We report a case with transient severe left ventricular dysfunction after intentional exposure to CO. The patient was early detected with an echocardiographic exam and treated with conventional treatment including high concentration of oxygen.
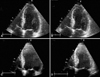
A 28-year-old man was admitted to our emergency room for altered mentality due to intentional exposure to CO. On his arrival, blood pressure was 104/80 mmHg, the pulse 126 beat per minute, axillary temperature 37.7℃ and the respirations were 32 breaths per minute. On blood analysis, AST/ALT 37/29 IU/L, CK 412 U/L, CK-MB 6.9 ng/mL, troponin I 0.96 ng/mL, N-terminal pro B-type natriuretic peptide 451.5 pg/mL, and CO-Hb 27.7%. The patient was intubated and treated with high concentration of oxygen therapy. A radiograph of the chest showed pulmonary edema and mild cardiomegaly (Fig. 1A). An electrocardiogram revealed sinus tachycardia of heart rate 120 per minute. Transthoracic echocardiogram showed global hypokinesia of left ventricle with severe systolic dysfunction (Fig. 2A and B). He was treated with diuretics, angiotensin converting enzyme inhibitor and urine alkalinization. Cardiac enzymes were elevated to CK 5,994 U/L, CK-MB 38.6 ng/mL, and troponin I 11.7 ng/mL on the third admission day. CK level was elevated to 15,951 U/L due to rhabdomyolysis and normalized with urine alkalinization. The follow-up chest radiograph showed normalized cardiac size and disappearance of pulmonary edema (Fig. 1B). The echocardiography taken after four days of treatment revealed normalized left ventricular systolic function (Fig. 2C and D). The patient discharged without any complication.
CO poisoning occurs after the inhalation of CO which is a toxic gas with colorless, odorless, tasteless, and non-irritating properties.2) CO is a product of combustion of organic matter with insufficient oxygen content, and is often produced in domestic or industrial settings by various materials including vehicles and other gasoline-powered machines, heaters, and cooking equipments. As a result, CO poisoning is the most common type of fatal poisoning world-widely.3)
CO is easily absorbed through the lungs and combines with hemoglobin to form CO-Hb in the blood and prevents binding with oxygen causing hypoxemia to anoxemia. Myoglobin and mitochondrial cytochrome oxidase are thought to be compromised. CO-Hb can revert to haemoglobin, but the recovery takes time because CO-Hb is fairly stable.2)
Patients may demonstrate varied clinical manifestations with different outcomes, even under similar exposure conditions.4) Inhaling even relatively small amounts can lead to hypoxic injury, neurological damage, and possibly death. One report concluded that CO exposure can lead to significant loss of lifespan after exposure due to damage to the heart muscle.5) Toxicity is also increased by several factors, including: increased activity and rate of ventilation, preexisting cerebral or cardiovascular disease, reduced cardiac output, anemia or other hematological disorders, decreased barometric pressure, and high metabolic rate.4) Symptoms of mild poisoning include headaches, vertigo, and flu-like effects; larger exposures can lead to significant toxicity of the central nervous system, heart, and even death.
Since the first report about CO-induced cardiac damage by Klebs in 1865, heart failure and myocardial ischemia have been described in the patients after acute exposure to the CO.1)6) After exposure to CO, several clinical manifestations have been reported, including arrhythmias and electrocardiographic alterations,1) acute myocardial infarction,7) pulmonary edema, and cardiogenic shock.8) Moreover, acute circulatory collapse and myocardial damage have been frequently observed in lethal cases. Patients with coronary artery disease are more susceptible to CO-induced cardiotoxicity.9)
Tachycardia is the most common finding among cardiocirculatory changes after acute CO exposure.10) It is usually considered as a compensatory response to systemic hypoxemia and decreased cardiac systolic function. Chest discomfort or pain can be resulted from myocardial ischemia or necrosis in the presence and in the absence of coronary artery disease.10-12) Shortness of breath and low blood pressure can be symptoms of cardiac dysfunction.13) In this patient, shortness of breath and tachycardia were noted at the time of admission and these symptoms suggesting the presence of cardiac dysfunction.
Electrocardiographic changes are usually nonspecific and may be a misleading index of severity in CO intoxication.1)8) The evaluation of cardiac markers can be of considerable diagnostic value in the presence of chest discomfort or ischemic electrocardiographic changes. Troponin I and troponin T have been successfully used in the diagnosis of CO-induced cardiotoxicity.5)14)
Echocardiography is a good screening tool for detection of CO-induced cardiotoxicity. Diffuse or segmental wall motion abnormality can be observed in patients with CO exposure. In clinical studies, echocardiography is more sensitive than electrocardiography in detecting CO-induced cardiac damage and more effective for severity assessment.8)15) In one study conducted by Satran et al.14) analyzed total 230 consecutive patients with intentional CO exposure. Of them, 53 patients were underwent echocardiographic examination and 57 percent of patients showed abnormal left ventricular function. Patients with global left ventricular dysfunction were younger (average age 43 years) with few cardiac risk factors and more severe CO poisoning. The proposed mechanism of global left ventricular dysfunction is myocardial stunning as a result of CO poisoning. Patients with regional wall motion abnormalities were older (average age 64 years) with a higher frequency of cardiac risk factors. The possible mechanism is unmasking of underlying coronary arterial disease by creating myocardial demand/supply mismatch. Usually, the left ventricular dysfunction was normalized with conventional treatment including high concentration of oxygen. In our case, global left ventricular systolic dysfunction was associated with severe CO poisoning and recovered after conventional therapy.
CO exposure may induce reversible or permanent cardiac damage. The conventional diagnostic tools routinely used to evaluate cardiac ischemia (clinical evaluation, and electrocardiography) appear inadequate to correctly assessment of CO induced cardiotoxicity in some cases. Thus more aggressive diagnostic approach with echocardiography should be considered particularly in severe cases and in patients with preexisting heart diseases (coronary arterial diseases and/or heart failure).
Figures and Tables
Fig. 1
The chest X-ray checked on the admission demonstrates mild cardiomegaly and pulmonary edema (A). After three days of treatment, the cardiac size is normalized and pulmonary edema is disappeared (B).

Fig. 2
The echocardiogram performed at admission shows global hypokinesia and severe left ventricular systolic dysfunction with ejection fraction of 33% (A: end-diastole, B: end-systole). With four days of conventional treatment including high concentration of oxygen, follow up echocardiography reveals normalized left ventricular function without wall motion abnormalities (C: end-diastole, D: end-systole).
References
1. Gandini C, Castoldi AF, Candura SM, Locatelli C, Butera R, Priori S, Manzo L. Carbon monoxide cardiotoxicity. J Toxicol Clin Toxicol. 2001. 39:35–44.

4. Raub JA, Mathieu-Nolf M, Hampson NB, Thom SR. Carbon monoxide poisoning--a public health perspective. Toxicology. 2000. 145:1–14.

5. Henry CR, Satran D, Lindgren B, Adkinson C, Nicholson CI, Henry TD. Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning. JAMA. 2006. 295:398–402.

6. Marius-Nunez AL. Myocardial infarction with normal coronary arteries after acute exposure to carbon monoxide. Chest. 1990. 97:491–494.

7. Anderson RF, Allensworth DC, DeGroot WJ. Myocardial toxicity from carbon monoxide poisoning. Ann Intern Med. 1967. 67:1172–1182.

8. McMeekin JD, Finegan BA. Reversible myocardial dysfunction following carbon monoxide poisoning. Can J Cardiol. 1987. 3:118–121.
9. Allred EN, Bleecker ER, Chaitman BR, Dahms TE, Gottlieb SO, Hackney JD, Pagano M, Selvester RH, Walden SM, Warren J. Shortterm effects of carbon monoxide exposure on the exercise performance of subjects with coronary artery disease. N Engl J Med. 1989. 321:1426–1432.

10. Thompson N, Henry JA. Carbon monoxide poisoning: poisons unit experience over five years. Hum Toxicol. 1983. 2:335–338.

11. Balzan MV, Cacciottolo JM, Mifsud S. Unstable angina and exposure to carbon monoxide. Postgrad Med J. 1994. 70:699–702.

12. Ebisuno S, Yasuno M, Yamada Y, Nishino Y, Hori M, Inoue M, Kamada T. Myocardial infarction after acute carbon monoxide poisoning: case report. Angiology. 1986. 37:621–624.

13. Diltoer MW, Colle IO, Hubloue I, Ramet J, Spapen HD, Nguyen N, Huyghens LP. Reversible cardiac failure in an adolescent after prolonged exposure to carbon monoxide. Eur J Emerg Med. 1995. 2:231–235.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download