Abstract
ST-segment elevation myocardial infarction (STEMI) is a disease decribed by typical chest pain, ST-segment elevation on eletrocardiogram, elevated cardiac enzymes, along with wall motion abnormality under echocardiographic findings, and it is caused by vulnerable plaques. However, stress induced cardiomyopathy (SICM) may show similar clinical symptoms, but specific echocardiographic findings (i.e. transient left ventricular regional wall motion abnormalities with peculiar apical ballooning appearance) and normal coronary angiography may differentiate it from STEMI. Therefore, one may mistake STEMI for SICM, and lead to serious error in diagnosis and treatment of the disease. We report a case of STEMI mimicking SICM, and suggest an idea to approach the patient with SICM.
Symptoms of ST-segment elevation myocardial infarction (STEMI) is caused by vulnerable plaque, and is described by typical chest pain, ST change under electrocardiogram (ECG), elevated cardiac enzymes, along with wall motion abnormality under echocardiographic findings. Although the incidence of stress-induced cardiomyopathy (SICM) is low according to Cangella et al.1) it shares similar clinical symptoms with STEMI except for echocardiographic findings. Under echocardiogram, the differential diagnoses are made, with SICM typically showing regional systolic dysfunction of the left ventricular walls with hypokinesis of the mid-apical segments and hyperkinesis of the basal segment.
However, rare cases of apical sparing and other wall motion involvement have also been cited.2) Thus, without precaution, these two diseases may be difficult to differentiate, and one may overlook the proper management of the two different diseases. We have experienced an STEMI patient showing echocardiographic findings consistent with SICM. The purpose of this report is to arouse clinicians about how important it is to make proper diagnosis and management in this circumstance.
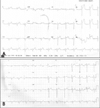
A 52-year-old woman visited our hospital complaining of continuous chest pain for 2 hours with nausea, and epigastric pain. After onset of chest pain, the initial ECG taken at private clinic showed mild ST-segment elevation on the lead I, aVL and tall T wave on the lead V4, and V5. Then she was immediately referred to our hospital due to sustained chest pain and ECG change (Fig. 1A). The patient was distressed by certain family trouble 3 days ago. She had a blood pressure of 120/70 mmHg, a pulse rate of 70 beats/min and a respiratory rate of 20 breaths/min. Physical examination was essentially normal. On the chest X-ray, her heart heart size was mildly enlarged with cardiothoracic ratio of 0.53. Complete blood count was within normal limits, electrolytes and thyroid function tests were also within normal limits. Cardiac enzymes were elevated with a troponin-T count of 1.10 ng/mL (normal: 0.00-0.10 ng/mL), and CK-MB of 13.09 ng/mL (normal: 0-5 ng/mL). The serum brain natriuretic peptide concentration was elevated to 991 pg/mL (normal 0-300 pg/mL) and serum norepinephrine was 153.7 pg/mL (normal 0-600 pg/mL). After admission to our hospital, her ECG was checked and echocardiogram was taken. ECG showed normalized ST segment and T waves (Fig. 1B), and echocardiographic findings showed regional systolic dysfunction of the left ventricular walls with hypokinesis of the mid-apical segments and hyperkinesis of the basal segments with left ventricular ejection fraction of 44.2% (Fig. 2). She was started with management according to SICM. She underwent a cardiac catheterization to rule out the possibility of coronary arterial stenosis, and started with heparinization and antiplatelet therapy. Coronary angiogram revealed normal right coronary artery (RCA). However, there was severe spastic nature of left coronary artery, especially at proximal left anterior descending (LAD) artery with typical chest pain (Fig. 3A). After administration of nitrate into the coronary artery, spasm was resolved (Fig. 3B) and fixed discrete lesion was shown at ostium of LAD artery at spidal view (Fig. 3C). On intravascular ultrasound exam, there was an 80% eccentric atheroma from ostium of left main coronary artery to ostium of LAD. A 3.5×13 mm sized sirolimus-eluting stent (Cypher™, Cordis, Johnson and Johnson Co. US) was inserted and 4.0×8 mm sized additional ballooning was done at the stent site. Then she was managed with calcium channel blockers, nitrate, antiplatelet agents and statin. She was discharged after symptomatic improvement. A subsequent echocardiogram performed after 2 weeks revealed complete resolution of the wall motion abnormalities (Fig. 2).
STEMI show diverse symptoms. It is difficult to differentiate SICM from STEMI by clinical symptoms in that they both share similar clinical manifestations. In 1990, Sato et al.3) first described "an acute cardiomyopathy characterized by acute but rapidly reversible, left ventricular systolic dysfunction in the absence of coronary artery disease which appeared to be triggered by intense psychological and physical stress." Despite of the increasing awareness of acute stress-induced myocardial dysfunction, the mechanism remains unknown. One possible mechanism is ischemia resulting from epicardial coronary arterial spasm. An alternative mechanism is microvascular spasm.4)5) According to Kurisu et al.6) 10% of SICM patients had spontaneous coronary vasospasm with catheter engagement and 67% had spasm in the acute period provoked by acetylcholine or ergonovine. These findings show that coronary artery spasm may be the culprit. Furthermore, if the spasm proceeds, acute myocardial infarction may result.7)8) A third possible mechanism of catecholamine-mediated myocardial stunning is direct myocyte injury.9) SICM is sometimes called aborted myocardial infarction10) and since medications that inhibit sympathetic activation show good results for medically manipulated rats with myocardial infarction.11) Therefore, it can be concluded that SICM and STEMI may share same or similar pathophysiologic mechanisms.
STEMI or SICM seems to favor patients with old age and stress is the trigger factor.12)13) The clinical characteristics of both STEMI and SICM are consistent across series and include the acute onset of chest pain, loss of consciousness, arrhythmia (ventricular fibrillation), cardiogenic shock, and myocardial rupture. There are acute myocardial infarction like changes (ST segment elevation, Q wave formation, and inverted T wave) on ECG and mild elevation of cardiac enzyme. Because excessive LV basal hyper-contractility lead to pressure overload and regional LV wall stress, this promotes secretion of brain natriuretic peptide.15-21) Their clinical manifestations are very similar to one another, but SICM differs from STEMI in that SICM shows normal coronary angiographic findings and it recovers to normal limits under natural course of the disease. Furthermore, echocardiographic findings of SICM show transient akinesis or dyskinesis of the left ventricular apical and mid-ventricular segments with regional wall-motion abnormalities extending beyond a single epicardial vascular distribution, but echocardiographic findings of STEMI show regional wall motion abnormality. Even though they share similar clinical manifestations, management and prognosis differs greatly.
In this report, provocative spasm study was not performed, but we suggest that there may also have been spasm in the both right and left coronary arteries, since there were global wall motion abnormality and catheter induced spasm on left coronary artery. Even if echocardiogra-phic findings suggest SICM, heparinization must be performed because STEMI cannot be ruled out. Cardiac catheterization may be performed early in the course to exclude acute coronary syndrome, but if cardiac catheterization cannot be performed early in the course, SICM patients might be treated according to STEMI.22)23) When a clinician meets SICM, and until final diagnosis is made, he or she must be aware of the possibility of STEMI. It is considered that such patients may be treated with calcium channel blocker initially, and beta blockers may not be used.
Figures and Tables
Fig. 1
Twelve-lead electrocardiogram revealed mild ST elevation on lead I, aVL and tall T-waves on lead V4, 5 (A). After admission to our hospital, ectrocardiogram showed normalized ST segment and T waves (B).
References
1. Cangella F, Medolla A, De Fazio G, Iuliano C, Curcio N, Salemme L, Mottola G, Agrusta M. Stress induced cardiomyopathy presenting as acute coronary syndrome: Tako-Tsubo in Mercogliano, Southern Italy. Cardiovasc Ultrasound. 2007. 5:36.

2. Derian W, Soundarraj D, Rosenberg MJ. Stress-induced cardiomyopathy: not always apical ballooning. Rev Cardiovasc Med. 2007. 8:228–233.
3. Sato H, Tateiski H, Uchida T. Kodama K, Haze K, Hon M, editors. Takotsubo type cardiomyopathy due to multivessel spasm. Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure (in Japanese). 1990. Tokyo: Kagakuhyouronsya;56–64.
4. Lacy CR, Contrada RJ, Robbins ML, Tannenbaum AK, Moreyra AE, Chelton S, Kostis JB. Coronary vasoconstriction induced by mental stress (simulated public speaking). Am J Cardiol. 1995. 75:503–505.

5. Sharkey SW, Shear W, Hodges M, Herzog CA. Reversible myocardial contraction abnormalities in patients with an acute noncardiac illness. Chest. 1998. 114:98–105.

6. Kurisu S, Sato H, Kawagoe T, Ischihara M, Shimatani Y, Nishioka K, Kono Y, Umemura T, Nakamura S. Takotsubo like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J. 2002. 143:448–455.

7. Yang NI, Hung MJ, Cherng WJ. Coronary artery spasm-related acute coronary syndrome in patients with coexisting spasm of angiographically normal coronary artery and fixed narrowing of the remaining vessels. Angiology. 2007. 58:156–160.

8. Romaqnoli E, Lanza GA. Acute myocardial infarction with normal coronary arteries: role of coronary artery spasm and arrhythmic complications. Int J Cardiol. 2007. 117:3–5.

9. Mann DL, Kent RL, Parsons B, Cooper G. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992. 85:790–804.

10. Khallafi H, Chacko V, Varveralis N, Elmi F. "Broken heart syndrome": catecholamine surge or aborted myocardial infarction? J Invasive Cardiol. 2008. 20:E9–E13.
11. Kolettis TM, Baltogiannis GG, Tsalikakis DG, Tzallas AT, Agelaki MG, Fotopoulos A, Fotiadis DI, Kyriakides ZS. Effects of dual endothelin receptor blockade on sympathetic activation and arrhythmogenesis during acute myocardial infarction in rats. Eur J Pharmacol. 2008. 580:241–249.

13. Wilbert-Lampen U, Leistner D, Greven S, Pohl T, Sper S, Völker C, Göthlin D, Plasse A, Knez A, Küchenhoff H, Steinbeck G. Cardiovascular events during World Cup soccer. N Engl J Med. 2008. 358:475–483.

14. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral Features of Myocardial Stunning Due to Sudden Emotional Stress. N Engl J Med. 2005. 352:539–548.

15. Chang KY, Jeon HK, Chae JS, Ihm SH, Seung KB, Youn HJ, Baek SH, Kim JH, Hong SJ, Choi KB. A novel cardiomyopathy mimicking acute myocardial infarction. Kor Circ J. 2002. 32:608–612.

16. Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic Review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004. 141:858–865.

17. Akashi YJ, Nakazawa K, Sakakibara M, Miyake F, Koike H, Sasaka K. The clinical features of takotsubo cardiomyopathy. QJM. 2003. 96:563–573.

18. Kim TS, Chu EH, Kang HH, Chun SW, Cho EJ, Kim JH. A Case of Reversal of Takotsubo Cardiomyopathy in Patient with Pheochromocytoma. J Cardiovasc Ultrasound. 2007. 15:50–54.

19. Kim DH, Bang DW, Ahn JH, Park SH, Oh HS, Yoon YJ, Hyon MS, Kim SK, Kwon YJ. Three Cases of Stress Induced Transient LV Dysfunction-Stress Induced Cardiomyopathy. J Kor Soc Echo. 2005. 13:83–86.

20. Chung JW, Kang MJ, Kim YH, Chang JH, Ha SI, Kim HJ, Koh YY, Chang KS, Hong SP. The Clinical Feature of Regional Wall Motion Abnormality on Apex of the Left Ventricle with Normal Coronary Angiogram. J Kor Soc Echo. 2005. 13:74–79.

21. Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic Review: Transient Left Ventricular Apical Ballooning: A Syndrome That Mimics ST-Segment Elevation Myocardial Infarction. Ann Intern Med. 2004. 141:858–865.

22. Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol. 2006. 48:1319–1325.
23. Fragmin and Fast Revascularisation During Instablility in Coronary Artery Disease Investigators. Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. Lancet. 1999. 354:708–715.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download