Abstract
Pneumopericardium is defined as the condition of presence of air in the pericardial space. It is associated with various etiologies such as chest trauma, infection or invasive procedures. We herein describe a case of cardiac tamponade associated with pneumopericardium. We diagnosed iatrogenic pneumopericardium by plain chest radiography and two-dimensional echocardiography. The patient was successfully treated by re-pericardiocentesis.
Pericardiocentesis is a procedure for drainage of pericardial effusion. Particularly, in patients with signs of cardiac tamponade, this procedure should be performed immediately to relieve tamponade and to collect specimens for diagnosis at the same time.1-3) Pericardiocentesis is a relatively safe technique and is rarely associated with serious complications including death.1)3-6) Here, we report a case with cardiac tamponade due to iatrogenic pneumopericardium following therapeutic pericardiocentesis.
A 91-year-old man visited the emergency room for dyspnea of NYHA functional class III for one day. He was diagnosed to have acute myocardial infarction and chronic kidney disease 7 months ago. At that time, echocardiography showed akinesia of anteroseptum with mild left ventricular (LV) systolic dysfunction (LV ejection fraction=49%) and small amount of pericardial effusion.
On his second visit, his vital signs were stable: blood pressure, 120/80 mmHg; pulse rate, 84 bpm; respiratory rate, 20/min; and body temperature, 36.8℃ However, physical examination showed acutely ill appearance, decreased breath sound on bilateral lower lung fields, neck vein engorgement, and pretibial pitting edema. Electrocardiography revealed sinus rhythm with complete right bundle branch block and low QRS voltage in limb leads. Laboratory study showed that serum creatinine was 2.4 mg/dL (normal range: 0.7-1.7 mg/dL) and serum pro-B-type natriuretic peptide was 26,700 pg/mL (normal range: <146 pg/mL). Electrocardiography demonstrated sinus rhythm with incomplete right bundle branch block and low voltage in limb leads. Chest X-ray revealed cardiomegaly (cardiothoracic ratio=0.8) and bilateral pleural effusions (Fig. 1A).
Two-dimensional echocardiography showed markedly increased amount of pericardial effusion with tamponade features including right atrium (RA) collapse and diastolic collapse of right ventricle (RV).
Emergent pericardiocentesis was performed by fluoroscopy-guided subxiphoidal approach. We removed 640 mL of serous fluid and placed a drainage catheter in the pericardial cavity. The catheter was connected to drainage bottle using a stopcock. There was minimal amount of residual pericardial fluid on echocardiography after pericardiocentesis. Soon after pericardiocentesis, dyspnea was relieved.
In the next morning, the drainage catheter was found to be removed from the patient's chest during sleeping. Two days later, he complained of progressive dyspnea on exertion and subsequently orthopnea at rest. His systolic blood pressure was about 90 mmHg which was 20 to 30 mmHg lower than that of post-pericardiocentesis. Follow-up chest X-ray showed cardiomegaly and "continuous diaphragm sign" which was implying visualization of central portion of diaphragm owing to presence of air in the pericardial cavity (Fig. 1B). Both decubitus views demonstrated thin parietal pericardium and air-fluid level in pericardial cavity, indicating hydropneumopericardium (Fig. 1B, C and D). Echocardiography revealed moderate amount of pericardial effusion with collapse of RA and RV and multiple bright echogenic spots swirling in the pericardial cavity, suggesting air microbubbles (Fig. 2).
He was underwent pericardiocentesis repeatedly to drain the pericardial fluid and air. On fluoroscopy, large multiple lumps of air moving in the pericardial cavity along the heart beats were observed (Fig. 3). When inserting the catheter into the pericardial space, blow-out sound of air leakage was heard. Dyspnea was relieved and blood pressure was normalized after the removal of large amount of air with additional 270 mL of fluid. Pericardial effusion was found to be tuberculous pericarditis and anti-tuberculous therapy was started.
Pneumopericardium is defined as the presence of the air in the pericardial cavity.7-9) It occurs more frequently in infants with respiratory distress syndrome, particularly in patients with mechanical ventilation.9)10) Common etiologies of pneumopericardium in adults are penetrating or blunt chest trauma, invasive diagnostic and therapeutic procedures to upper alimentary tracts or airway, barotrauma, formation of fistula from intestine or respiratory tracts to pericardium, and infection with gas-forming organisms.7)8)10-12) It can be associated with pneumothorax, pneumomediastinum or subcutaneous emphysema.9)10)
Since patients with pneumopericardium do not show any specific symptoms, early detection of the disease is usually not easy.10) Mill-wheel murmur produced by churning movement of the heart in a pericardial cavity is a pathognomic finding on auscultation.7)10) Presence of pneumopericardium can be ensured by plain chest radiography, computed tomography or echocardiography.7)10) Diagnosis only using chest X-ray may be difficult. Chest X-ray may show air separating pericardium from the heart or "continuous diaphragm sign" that indicates the presence of air within pericardium making normally invisible parts of central diaphragm visible in continuation with both hemidiaphragms.10) Computed tomography can offer confirmation of the diagnosis in obscure cases and additional information about associated lesions.9) As ultrasound beam can not easily pass through air, echocardiography is not routinely used for diagnosis of pneumopericardium. However, "air gap sign" that means the cyclic appearance of air in the pericardium or visualization of swirling air bubbles (swirling bubbles sign) or air-fluid interface in the pericardial cavity can be revealed on two-dimensional or M-mode echocardiography.13-15)
Differential diagnosis of pneumopericardium includes diseases that present chest pain or sudden dyspnea, especially pneumomediastinum.10) In pneumopericardium, the extent of air is superiorly confined to the level of pulmonary artery and ascending aorta, whereas, in pneumomediastinum, it extends up to superior mediastinum and neck.10) Prognosis is dependent upon combined etiologies. In most circumstances, they undergo benign clinical course of spontaneous resolution with bed rest, symptomatic care, and close monitoring.7)10) However, immediate drainage is mandatory for the cases that are carrying signs of tamponade or infection.7)10) Further surgical correction for combined etiology is also needed.9)11)
Pericardiocentesis is the procedure that is performed with diagnostic and therapeutic purposes in the cases of pericardial effusion, particularly in the patients with signs of tamponade.1)2) Complications related to pericardiocentesis develop in 1-3% and include injuries of right ventricle or coronary arteries, arrhythmias, injury of intraabdominal organs, hypotensive vagal reaction, pneumothorax, hemopericardium or death.1)6) Several authors14)16) have reported cases of pericardiocentesis-associated pneumopericardium and suggested that the mechanisms is a leakage of drainage system or formation of communication between pleura and pericardium.
This patient underwent pericardiocentesis to remove large amount of pericardial effusion and the catheter was accidentally removed from the drainage system, resulting in progressive worsening of dyspnea and more drop of blood pressure. The patient was diagnosed to have hydropneumopericardium by chest X-ray and echocardiography. Patient's clinical condition was thought to be deteriorated due to accumulation of heavy air and re-pericardiocentesis was done. Pneumopericardium following pericardiocentesis occurs infrequently, but it may be fatal without watchful surveillance and early detection. In terms of pericardiocentesis, meticulous procedural technique and careful observation after the procedure are necessary.
Figures and Tables
 | Fig. 1A: Chest X-ray showed cardiomegaly with bilteral pleural effusions at admission. B: Chest PA on third hospital day demonstrated cardiomegaly with the line separating pericardium from the heart (black arrow heads) and "continuous diaphragm sign" (white arrow heads) implying presence of air in the pericardial cavity, pneumopericardium. C-D: Both decubitus views revealed thin parietal pericardium (white arrows) and air-fluid level (black arrows) in the pericardial cavity, which suggests hydropneumopericardium. |
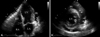 | Fig. 2Two-dimensional echocardiography [A: apical four-chamber view, B: parasternal short axis view, papillary muscle level] showed pericardial effusion (PE) with the "swirling bubbles sign" that indicated multiple unusual tiny echo-bright spots (white arrows) in pericardial cavity, suggesting formation of air microbubbles through continuous shaking of the air-fluid surface with heart beats. LV: left ventricle, LA: left atrium, RA: right atrium. |
References
1. Guberman BA, Fowler NO, Engel PJ, Gueron M, Allen JM. Cardiac tamponade in medical patients. Circulation. 1981. 64:633–640.

3. Han SW, Kim KS, Hur SH, Hyun DW, Kim YN, Kim KB. Efficacy of 2-dimensional contrast echocardiography during pericardiocentesis. J Kor Soc Echocardiogr. 1996. 4:80–84.

4. Tsang TS, Enriquez-Sarano M, Freeman WK, Barnes ME, Sinak LJ, Gersh BJ, Bailey KR, Seward JB. Consecutive 1127 therapeutic echocardiographically guided pericardiocentesis: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002. 77:429–436.

6. Bernal JM, Pradhan J, Li T, Tchokonte R, Afonso L. Acute pulmonary edema following pericardiocentesis for cardiac tamponade. Can J Cardiol. 2007. 23:1155–1156.

7. Bhindi R, Rees DM. Pneumopericardium masquerading as an acute myocardial infarction. Int J Cardiol. 2007. 114:e83–e84.

8. Albrecht CA, Jafri A, Linville L, Anderson V. Cocaine-induced pneumopericardium. Circulation. 2000. 102:2792–2794.

9. Rashid MA, Wikström T, Örtenwall P. Pneumopericardium and pneumoperitoneum after penetrating chest injury. Eur J Surg. 1999. 165:278–279.

10. Brander L, Ramsay D, Dreier D, Peter M, Graeni R. Continuous left hemodiaphragm sign revisited: a case of spontaneous pneumopericardium and literature review. Heart. 2002. 88:e5.
11. Müller AMS, Betz MJ, Kromeier J, Ghanem NA, Geibel A, Imdahl A, Frydrychowicz AP. Acute pneumopericardium due to intestine-pericardial fistula. Circulation. 2006. 114:e7–e9.
12. van Haren FMP, Borstlap A, Fouraine N. Tension pneumopericardium in Hodgkin's diasease. Circulation. 2006. 114:e77–e79.
13. Reid CL, Chandraratna AN, Kawanishi D, Bezdek WD, Schatz R, Nanna M, Rahimtoola SH. Echocardiographic detection of pneumomediastinum and pneumopericardium: the air gap sign. J Am Coll Cardiol. 1983. 1:916–921.

14. Bedotto JB, McBride W, Abraham M, Taylor AL. Echocardiographic diagnosis of pneumopericardium and hydropneumopericardium. J Am Soc Echocardiogr. 1988. 1:359–361.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download