Abstract
The purpose of the present study was to assess the effects of early initiation of statin treatment on midterm major adverse cardiac events (MACEs) after acute myocardial infarction (AMI). Between October 2005 and December 2006, 621 AMI patients (439 ST-segment and 182 non-ST-segment elevation MI) were registered and followed prospectively. Early initiation of statin treatment was defined as prescription of statins during hospitalization (n=545), and the control group was not prescribed statins (n=76). During the 6-month follow-up, 22 patients died: 7 patients from the control group (9%) and 15 patients from the statin group (3%) (p=0.004). Six-month MACEs (including cardiac death, MI, and target vessel revascularization) occurred less frequently in the statin group than in the control group (14% vs. 24%, p=0.034). Baseline high-sensitivity C-reactive protein (hs-CRP) was significantly higher in patients who experienced cardiac death than in patients without cardiac death (12.8±10.2 mg/dl vs. 2.1±3.0 mg/dl, p<0.001). Multivariate analysis showed that early statin therapy and the baseline hs-CRP level were independent predictors of 6-month mortality [odds ratio (OR)=0.078, 95% CI: 0.008~0.808, p=0.032, and OR=1.314, 95% CI: 1.149~1.501, p<0.001, respectively). High inflammatory status is associated with poor prognosis, and early initiation of statin treatment after AMI could decrease the mid-term mortality rate.
Hydroxymethylglutaryl coenzyme A reductase inhibitor (statin) therapy lowers mortality and morbidity in coronary artery disease and other atherosclerotic vascular disease, as evidenced by multiple large-scale clinical trials.1-4 Statins inhibit mevalonate synthesis and are effective at lowering low-density lipoprotein (LDL)-cholesterol. Beyond lowering lipids, statins have favorable effects on vascular inflammation,5,6 endothelial function,7,8 and platelet adhesion and thrombosis.9 The beneficial effects of statins mostly rely on their anti-inflammatory properties.10
Previous studies of patients with acute myocardial infarction (AMI) have suggested that early initiation of statins is associated with a significantly lower occurrence of early complications, a reduced infarct size, and better in-hospital survival.11,12 However, to the best of our knowledge, few data are available regarding the effects of early initiation of statin treatment on midterm clinical outcome including cardiac mortality. In the present study, we assessed the effects of early initiation of statin treatment on midterm clinical events after AMI in a real-world setting.
From October 2005 to December 2006, 621 patients with AMI (439 ST-segment elevation MI and 182 non-ST-segment elevation MI) were registered and followed prospectively at Chonnam National University Hospital, Gwangju, Korea, and the analysis was performed retrospectively. Inclusion criteria were the presence of a non-ST-segment elevation MI or an ST-segment elevation MI. The presence of ST-segment elevation MI was determined by >30 min of continuous chest pain, a new ST-segment elevation ≥2 mm on at least two contiguous electrocardiographic leads, and creatine kinase-MB >3 times normal. The presence of non-ST-segment elevation MI was diagnosed by chest pain and a positive cardiac biochemical marker without new ST-segment elevation. We excluded patients with prior MI, subacute or late stent thrombosis, restenosis after stenting, coronary artery bypass graft failure, hepatic or renal dysfunction (alanine aminotransferase and aspartate aminotransferase >2 times normal, creatinine >1.5 mg/dl), or current use of any lipid-lowering drugs. Early initiation of statin treatment was defined as prescription of statins during hospitalization (statin group: 545 patients), and the control group was not prescribed statins during hospitalization or at discharge (nonstatin group: 76 patients). The hospital records of all patients were reviewed to obtain information on clinical demographics and medical history. Risk factors were reviewed via retrospective chart review.
All laboratory values were measured after the patients had fasted overnight. The serum levels of total cholesterol, LDL-cholesterol, high-density lipoprotein (HDL)-cholesterol, and triglyceride were measured by standard enzymatic methods. High-sensitivity C-reactive protein (hs-CRP) was analyzed turbidimetrically with sheep antibodies against human CRP; this has been validated against the Dade-Behring method.13
Infarct-related arteries were identified by using a combination of electrocardiographic findings, left ventricular wall motion abnormalities on left ventricular angiogram or echocardiogram, and coronary angiographic findings. Coronary angiogram was performed in 588 patients. Of these patients, 544 patients were treated with stent implantation (442 patients with drug-eluting stents and 102 patients with bare metal stents), and balloon angioplasty was done in 44 patients. Stent implantation was performed as previously described.14 Patients were treated with aspirin [300 mg loading before percutaneous coronary intervention (PCI) and 100~200 mg daily, indefinitely] and clopidogrel (300 or 600 mg before PCI and 75 mg daily continued for at least 6 months). Glycoprotein IIb/IIIa inhibitors were used at the operator's decision.
The primary end point was occurrence of cardiac death during the 6-month follow-up. The secondary end point was the occurrence of the composite of cardiac death, MI, and target vessel revascularization during the 6-month follow-up. All deaths were considered to be of cardiac origin unless a noncardiac origin was established clinically or at autopsy. MI was defined by the presence of recurrent ischemic symptoms or electrocardiographic changes accompanied by a creatine kinase level that was more than twice the upper limit of the normal range or >50% higher than the value during the index hospitalization (with an elevated MB isoform level). Target vessel revascularization included bypass surgery or repeat PCI of the target vessels.
The Statistical Package for the Social Sciences (SPSS) for Windows, version 15.0 (Chicago, Illinois) was used for all analyses. Continuous variables were presented as the mean±SD; comparisons were conducted by Student's t-test or the nonparametric Wilcoxon test if the normality assumption was violated. Discrete variables were presented as percentages and relative frequencies; comparisons were conducted by chi-square statistics or Fisher's exact test as appropriate. Logistic regression analysis was used to identify the independent predictors of 6-month cardiac mortality. A p value <0.05 was considered statistically significant.
There were no significant differences in patient demographics or medications except for statin between the groups. The levels of LDL-cholesterol and hs-CRP were almost the same between the two groups (Table 1).
There were no significant differences in baseline coronary angiographic findings and procedural results between the two groups (Table 2). A total of 76% in the statin group and 78% in the nonstatin group had complex lesions, and 51% in the statin group and 53% in the nonstatin group had multivessel disease. Stents were used in 93% of the patients. Bare-metal stents were used in 18% and drug-eluting stents were used in 82% of the patients. The occurrence of post-PCI no-reflow phenomenon [which was defined as post-PCI thrombolysis in myocardial infarction (TIMI) flow grade 0, 1, and 2] was not significantly different between the groups.
Follow-up data were available for all patients. During the 6-month follow-up, 22 patients died (4%), including 7 patients from the control group (9%) and 15 patients from the statin group (3%) (p=0.004). Six-month major adverse cardiac events (including cardiac death, MI, and target vessel revascularization) occurred less frequently in patients who had taken early statin therapy than in patients who had not taken statin therapy (Fig. 1).
The patients who experienced cardiac death were older and had a higher Killip class on admission than did the patients without cardiac death. The patients who experienced cardiac death had more atrial fibrillation and ventricular tachyarrhythmia and had more deteriorated left ventricular function on admission than did the patients without cardiac death. Serum creatinine was significantly higher, creatinine clearance was significantly lower, and the HDL-cholesterol level was significantly lower in the patients who experienced cardiac death than in the patients without cardiac death. Baseline hs-CRP was significantly higher in the patients who experienced cardiac death than in the patients without cardiac death (Table 3). There were no significant differences in types or daily dosages of statins used between the patients who experienced cardiac death and those who did not.
There were no significant differences in the infarct-related artery, ACC/AHA lesion type, or baseline or post-PCI TIMI flow grade between the groups. However, multivessel disease was more frequently observed in the patients who experienced cardiac death than in those who did not (Table 4).
Multiple logistic regression analysis was performed to determine independent predictors of 6-month cardiac mortality. The following variables were tested (all with p<0.2 in the univariate analysis): early initiation of statins, age, hypertension, smoking, Killip class III/IV, atrial fibrillation, ventricular tachyarrhythmia, ejection fraction, creatinine clearance, total cholesterol, HDL-cholesterol, hs-CRP, multivessel disease, baseline TIMI flow grade, and stent use. Early initiation of statin therapy and baseline hs-CRP level were the independent predictors of 6-month mortality [odds ratio (OR)=0.078, 95% CI: 0.008-0.808, p=0.032, and OR=1.314, 95% CI: 1.149-1.501, p<0.001, respectively).
The results of this analysis demonstrate that high inflammatory status is associated with poor prognosis, and 6-month major adverse cardiac events including cardiac death, MI, and target vessel revascularization occurred less frequently in patients who had taken early statin therapy than in patients who had not taken statin therapy.
Statins inhibit mevalonate synthesis and are effective at lowering LDL-cholesterol. Beyond lowering lipids, statins have favorable effects on vascular inflammation,5,6,15 endothelial function,7,16 platelet adhesion, and thrombosis.9 Multiple studies have shown that statins lower mortality and morbidity in coronary artery disease and other atherosclerotic vascular disease.1,4,17 The beneficial effects of statins, beyond their lipid-lowering action, mostly rely on their anti-inflammatory properties.10
Inflammation plays an important role in both atherogenesis and atherothrombotic events, and hs-CRP has been associated with increased risk for coronary artery disease.13 hs-CRP measurement has been recommended for some patients to refine risk assessment because hs-CRP levels have been shown to provide additional predictive information beyond traditional risk factors such as LDL-cholesterol.18,19
Several studies have evaluated the effects of early statin therapy in patients with acute coronary syndrome. There is some controversy over the effects of early initiation of statin treatment in patients with acute coronary syndromes.11,12,20-24 Newby et al.20 reported that there was no relationship between early initiation of statin therapy and improved clinical outcomes. Briel et al.21 reported that initiation of statin therapy within 14 days of onset of acute coronary syndrome did not reduce death, MI, or stroke up to 4 months. In contrast, Stenestrand et al.11 reported that early initiation of statin treatment in patients with AMI is associated with reduced 1-year mortality. Hulten et al.22 reported that early, intensive statin therapy reduced death and cardiovascular events after 4 months of treatment. Chang et al.23 reported that statin therapy prior to PCI may reduce peri-PCI myonecrosis and late cardiac events.
In the present study of 621 AMI patients, baseline hs-CRP was significantly higher in patients with cardiac death than in patients without cardiac death, and 6-month major adverse cardiac events (including cardiac death, MI, and target vessel revascularization) occurred less frequently in patients who had taken early statin therapy than in patients who had not taken statin therapy. The mechanism of benefit of early initiation of statin treatment in reducing cardiac mortality is not well elucidated. Recent study suggests that statins have pleiotropic effects apart from their serum lipid-lowering effect in humans. One of the major target organs for the effects of statins is the vascular endothelium, which plays an important role in the development of atherosclerosis and angiogenesis. Numerous recent studies have shown that the statins' cholesterol-independent vascular effects appear to involve directly restoring or improving endothelial function by increasing NO production, promoting re-endothelialization after arterial injury, and inhibiting inflammatory responses within the vessel wall that are thought to contribute to atherosclerosis.25,26 These numerous pleiotropic effects of statins may start before their lipid-lowering effects.27
There are several limitations to be mentioned. First, this single-center study included only a small number of patients. Second, we did not assess the differences in clinical outcomes according to the kinds of statins and the dosages of statins used. Third, we included all AMI patients regardless of treatment methods (PCI or medical therapy). However, treatment methods were not an independent predictor of mortality in the present study.
In conclusion, in this analysis of 621 AMI patients, high inflammatory status was associated with poor prognosis, and early initiation of statin treatment after AMI could decrease the midterm mortality rate.
Figures and Tables
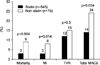 | Fig. 1Incidences of mortality, myocardial infarction (MI), target vessel revascularization (TVR), and total major adverse cardiac events (MACE) in the statin group and nonstatin group at the 6-month follow-up. |
Table 1
Patient demographics, medications, and lipid profiles

STEMI, ST-segment-elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; LDL, low-density lipoprotein-cholesterol; HDL, high-density lipoprotein-cholesterol; hs-CRP, high-sensitivity C-reactive protein.
Acknowledgements
This study was supported by a grant of the Korea Healthcare technology R&D project (A084869), Ministry for Health, Welfare & Family Affairs, Republic of Korea.
References
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994. 344:1383–1389.
2. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998. 339:1349–1357.
3. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo controlled trial. Lancet. 2002. 360:7–22.
4. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. Cholesterol and Recurrent Events Trial investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996. 335:1001–1009.

5. Strandberg TE, Vanhanen H, Tikkanen MJ. Effect of statins on C-reactive protein in patients with coronary artery disease. Lancet. 1999. 353:118–119.

6. Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998. 98:839–844.

7. Dupuis J, Tardif JC, Cernacek P, Théroux P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. The RECIFE (reduction of cholesterol in ischemia and function of the endothelium) trial. Circulation. 1999. 99:3227–3233.

8. Takayama T, Wada A, Tsutamoto T, Ohnishi M, Fujii M, Isono T, et al. Contribution of vascular NAD(P)H oxidase to endothelial dysfunction in heart failure and the therapeutic effects of HMG-CoA reductase inhibitor. Circ J. 2004. 68:1067–1075.

9. Lacoste L, Lam JY, Hung J, Letchacovski G, Solymoss CB, Waters D. Hyperlipidemia and coronary disease: correction of the increased thrombogenic potential with cholesterol reduction. Circulation. 1995. 92:3172–3177.
10. Libby P, Aikawa M. Mechanisms of plaque stabilization with statins. Am J Cardiol. 2003. 91:4B–8B.

11. Stenestrand U, Wallentin L. Swedish Register of Cardiac Intensive Care (RIKS-HIA). Early statin treatment following acute myocardial infarction and 1-year survival. JAMA. 2001. 285:430–436.

12. Fonarow GC, Wright RS, Spencer FA, Fredrick PD, Dong W, Every N, et al. National Registry of Myocardial Infarction 4 Investigators. Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am J Cardiol. 2005. 96:611–616.

13. Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J, et al. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem. 2001. 47:418–425.

14. Walter DH, Schächinger V, Elsner M, Dimmeler S, Zeiher AM. Platelet glycoprotein IIIa polymorphism and risk of coronary stent thrombosis. Lancet. 1997. 350:1217–1219.

15. Park SY, Kwak JJ, Park SH. Dose dependent changes of lipid profiles, IL-6 and CRP in unstable angina patients after aimvastatin therapy. Korean Circ J. 2003. 33:663–670.

16. Son JW, Koh KK. Effects of Statins on Endothelium : Vasomotor Function, Inflammation,and Hemostasis. Korean Circ J. 1999. 29:1016–1031.

17. Hong YJ, Jeong MH, Lim JH, Park HW, Kim HG, Park OY, et al. The prognostic significance of statin therapy according to the level of C-reactive protein in acute myocardial infarction patients who underwent percutaneous coronary intervention. Korean Circ J. 2003. 33:891–900.

18. Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. 2004. 116:Suppl 6A. 9S–16S.

19. Torres JL, Ridker PM. Clinical use of high sensitivity C-reactive protein for the prediction of adverse cardiovascular events. Curr Opin Cardiol. 2003. 18:471–478.

20. Newby LK, Kristinsson A, Bhapkar MV, Aylward PE, Dimas AP, Klein WW, et al. SYMPHONY and 2nd SYMPHONY Investigators. Sibrafiban vs Aspirin to Yield Maximun Protection From Ischemic Heart Events Post-acute Coronary Syndromes. Early statin initiation and outcomes in patients with acute coronary syndromes. JAMA. 2002. 287:3087–3095.

21. Briel M, Schwartz GG, Thompson PL, de Lemos JA, Blazing MA, van Es GA, et al. Effects of early treatment with statins on short-term clinical outcomes in acute coronary syndromes: a meta-analysis of randomized controlled trials. JAMA. 2006. 295:2046–2056.

22. Hulten E, Jackson JL, Douglas K, George S, Villines TC. The effect of early, intensive statin therapy on acute coronary syndrome: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006. 166:1814–1821.

23. Chang SM, Yazbek N, Lakkis NM. Use of statins prior to percutaneous coronary intervention reduces myonecrosis and improves clinical outcome. Catheter Cardiovasc Interv. 2004. 62:193–197.

24. Nishino M, Hoshida S, Kato H, Egami Y, Shutta R, Yamaguchi H, et al. Preprocedural statin administration can reduce thrombotic reaction after stent implantation. Circ J. 2008. 72:232–237.

26. Bao N, Minatoguchi S, Kobayashi H, Yasuda S, Kawamura I, Iwasa M, et al. Pravastatin reduces myocardial infarct size via increasing protein kinase C-dependent nitric oxide, decreasing oxyradicals and opening the mitochondrial adenosine triphosphate-sensitive potassium channels in rabbits. Circ J. 2007. 71:1622–1628.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download