Introduction
Coronary restenosis in stents has an incidence of between 20% and 30% with the use of bare metal stents (BMS) and occurs mainly during the first 6 months after stent implantation.1 In-stent restenosis (ISR) in BMS is caused by excessive neointimal hyperplasia and late regression. Pathophysiological specimens in humans have shown that neointima is formed by variable amounts of proliferating smooth muscle cells, macrophages, and inflammatory cells embedded in a matrix rich in water. The surface of the neointima is covered by endothelial cells, which play a critical role in preventing stent thrombosis. Whereas reendothelialization is usually complete in young animals in less than 1 month, it may take several months to be completed in humans. The thrombogenicity of stented lesions decreases as time goes on. Therefore, the occurrence of ISR within 1 year after BMS implantation is relatively low.2
However, we experienced the case of a 72-year-old male with unstable angina pectoris 7 years after BMS implantation. In Korea, no case of very late-ISR (VL-ISR) has been reported. Because the clinical presentation of patients who develop VL-ISR with BMS implantation has not been well characterized, this case and the accompanying illustrations should be of interest to both cardiologists and medical doctors.
Case Report
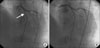
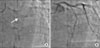
A 72-year-old male with no known history of diabetes mellitus, hypertension, or dyslipidemia presented to our hospital with increasing exertional dyspnea lasting 2 weeks. His initial blood pressure was 100/70 mm Hg and his pulse rate was 80 beats per minute. The electrocardiogram on admission showed T wave inversion in lead V2-6, left axis deviation, and left anterior fascicular block. Cardiac markers including CK-MB and troponin were normal. Echocardiography revealed akinesia of the anterior, anteroseptal wall and hypokinesia of the inferior, infero-septal wall. He had undergone percutaneous coronary intervention (PCI) with 3.0×15 mm BMS (Tsunami, Terumo, Europe NV, Belgium) in the mid-left anterior descending artery (LAD) in July 2002 at another hospital (Fig. 1). He had taken aspirin, cilostazol, and statin medication for 2 months after the intervention, and afterward aspirin medication. A follow-up angiogram performed 5 years later revealed no ISR in the m-LAD (Fig. 2).
Coronary angiography showed total occlusion in the proximal- to distal-LAD with contralateral collateral flow from the right coronary artery. We implanted a 3.0×20 mm drug eluting stent (DES) (Taxus, Boston Scientific, USA) for the proximal-LAD, a 2.75×20 mm DES (Taxus, Boston Scientific, U.S.A) for the mid-LAD, and a 2.75×24 mm DES (Taxus, Boston Scientific, USA) for the distal-LAD (Fig. 3). Because the patient underwent percutaneous coronary intervention, he did not show cardiogenic symptoms. He was discharged with astrix, Plavix, and follow-up in the outpatient department.
Discussion
Some cases of BMS ISR present as unstable angina requiring hospitalization or acute MI (non-ST-segment elevation MI and ST-segment elevation MI). ISR may play a negative role in the long-term survival of patients and may result in mortality in patients treated with BMS.3 Our case was associated with exertional dyspnea due to total occlusion of the stented lesion. Owing to the patient's normal cardiac enzymes, unchanged ST segment, presence of collateral flow, and absence of chest pain, we diagnosed our case as VL-ISR then acute stent thrombosis. Although collateral flow developed, untreated total occlusive ISR may result in a bad prognosis.
VL-ISR occurs rarely. BMS ISR is reported within 6 months after stent implantation. This phenomenon is related to re-endothelial dysfunction of the stented lesion. The surface of the neointima is covered by endothelial cells, which play a critical role in preventing stent thrombosis. Many unpredictable causes of neointimal hyperplasia after BMS implantation may disrupt good distal flow. First, tissue inside the stented lesion, which is covered by the intimal layer, has been demonstrated to be rich in the highly thrombogenic tissue factor. This intense tissue response with its thrombogenic nature may provide the substrate for the development of an ACS, particularly when the structure and function of the intimal layer is somehow altered.3 Second, the arterial injury from stent implantation may serve as a nidus that accumulates smooth muscle cells within the neointima and expands plaque by an increase in the extracellular matrix. Atheromatous plaque triggers lumen narrowing and brings on symptoms of ischemia.4 Third, intimal dissection at the stent edges precede re-endothelial dysfunction and plaque rupture.5 Finally, underlying disease may also be associated with ISR owing to atherosclerotic change. Diabetes mellitus, hypertension, dyslipidemia, and smoking are traditional risk factors for endothelial dysfunction and atherosclerosis.6
Clinicians should be concerned about the possibility of ISR in patients who have undergone BMS implantation and evaluate neointima hyperplasia balancing. The underlying disease should be controlled in these patients, and the patients should quit smoking to prevent mortality and morbidity due to ISR. Because the occurrence of BMS ISR cannot be easily predicted, DES should be strongly considered and implanted in a widespread manner with a combination of anti-platelet and statin agents.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download