Abstract
Houttuynia cordata (H. cordata) has anti-inflammatory and anti-bacterial activities. Staphylococcus aureus (S. aureus)-producing lipoteichoic acid (LTA) has been implicated in inflammatory and immunologic responses in patients with atopic dermatitis (AD). The purpose of this study was to determine the potential of H. cordata as a novel anti-inflammatory agent for AD by evaluating the anti-inflammatory activity of H. cordata water extract on LTA-induced inflammation in dermal fibroblasts (DFs). The anti-inflammatory activity of H. cordata was evaluated by studying the inflammatory markers tumor necrosis factor-α (TNF-α) and cyclooxygenase-2 (COX-2). As shown by MTT assay and COX-2 immunoblot, H. cordata was not toxic to DFs in doses up to 20µg/ml. In RT-PCR and Western blot analysis, LTA up-regulated TNF-α (mRNA) and COX-2 (protein) at doses of 5~20µg/ml in a dose-dependent manner. Next, DFs were treated with LTA (10µg/ml) in the absence (control) or presence of H. cordata for 24 h. The level of LTA-induced TNF-α mRNA was suppressed by H. cordata (20µg/ml) by up to 40% compared with the level in LTA-treated control samples. Under the same conditions, the level of LTA-induced COX-2 protein was suppressed by H. cordata treatment by up to 52% compared with the level in LTA-treated control samples. When DFs were treated with TNF-α (10µg/ml) for 24 h, TNF-α-induced COX-2 expression was down-regulated by H. cordata treatment to 65% of that in control samples. In conclusion, H. cordata has anti-inflammatory activity against LTA-induced inflammation in DFs, in part by blocking the TNF-α pathway.
Houttuynia cordata (H. cordata) is a traditional medicinal plant that is used in herbal preparations in Asian countries to relieve inflammatory conditions. H. cordata has been shown to have strong anti-inflammatory activity in a pleurisy model and in ear edema.1 H. cordata has been used as an anti-inflammatory agent for treating atopic dermatitis (AD) and acne in traditional Korean herbal medicine.
With respect to the mechanism by which AD inflammation is induced, Staphylococcus aureus has been widely studied for its active roles in inflammatory and immunologic responses against AD.2 Lipoteichoic acid (LTA) acts as an adhesion molecule for many Grampositive bacteria, including staphylococci, streptococci, and pneumococci.3 Recently, LTA was extensively studied in relation to the pathogenic role of S. aureus in AD inflammation.4
S. aureus cell walls are composed of highly crosslinked peptidoglycans (PEGs) decorated with teichoic acid (TA) polymers, which are also linked to plasma membrane phospholipids, of which LTA and PEG are the major surface components.5 TA or related glycopolymers play crucial roles in bacterial survival under suboptimal conditions and in other basic cellular processes.3,4 In AD, LTA induces IL-5 production from peripheral blood mononuclear cells (PBMCs) and AD-like skin lesions with the T helper type 2 (Th2) response, including the production of IL-4 and IL-5.5
Tumor necrosis factor (TNF)-α has a number of pro-inflammatory effects in skin inflammation and therefore has been studied extensively as a therapeutic target in treating TNF-α-mediated skin disorders, such as AD and psoriasis.6 In skin affected by AD, local tissue expression of pro-inflammatory cytokines and chemokines has focused on epidermal keratinocytes (KCs), T lymphocytes, dendritic cells, macrophages, mast cells, and eosinophils.7 S. aureus induces the production of major pro-inflammatory cytokines, including TNF-α, interleukin-1 (IL-1), IL-6, and IL-8 from KCs in the epidermis or from PBMCs, which are infiltrated into the dermis.8,9
However, dermal fibroblasts (DFs) have been reported to be another source of TNF-α production by various external stimuli.10-12 In this study, we determined whether DFs can be another source of production following stimulation by LTA. COX-2 is also a good biomarker for inflammation in various tissues, including skin, as it has been widely studied in relation to tissue inflammation, wounds or ulcer healing, and carcinogenesis.13 In this study, we used TNF-α and COX-2 as representative inflammatory markers
The purpose of the present study was to evaluate the anti-inflammatory activity of water extract of H. cordata against LTA-induced inflammation in DFs, with the prospect of developing a novel anti-inflammatory agent for AD in the future.
H. cordata leaves were collected from the Gwangju area in South Korea. The water extract was obtained by boiling 100 g of H. cordata leaves in 1 L of distilled water for 1 h. The extract was filtered and was concentrated by lyophilization. The extract yield from the fresh leaves was approximately 4.5%. H. cordata water extract powder was stored at ??0℃ until used in the experiments.
Intact human skin from circumcised foreskin was obtained after informed consent according to the guidelines of the local Ethics Committee of Chonnam National University Hospital Institutional Review Board and the Declaration of Helsinki Principles. The skin was cut into small pieces and was then treated with 2.4 U/ml dispase (Boehringer Mannheim, Ingelheim, Germany) for 30 min at 37℃. The dermal tissues were separated from the epidermis and minced into small pieces with iris scissors, and primary DFs were obtained by culturing the tissue in DMEM supplemented with 10% fetal bovine serum and antibiotics (100 U/ml penicillin and 100µg/ml streptomycin) in a 5% CO2 incubator. DFs from passages 2 to 4 were used for all experiments. The primary cultured cells were confirmed to be DFs by immunostaining with vimentin (Fig. 1).
To determine cell viability, the cells were seeded into 96-well plates and treated with H. cordata extracts for 24 h. We used a colorimetric 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide (MTT) kit (Chemicon International, Inc., Billerica, MA, USA) for determining cell survival and proliferation, according to the manufacturer's instructions. Briefly, 10 ml of MTT solution was added to each well (0.5 mg/ml), and the plates were incubated at 37℃ for 2 h. The resulting formazan crystals were dissolved with 100 ml of dimethylsulfoxide (DMSO) and absorbance was read at 570 nm in an ELISA reader (Molecular Devices, Sunnyvale, CA, USA). In this study, LTA from S. aureus was purchased from Sigma Chemical Company (St. Louis, MO, USA). From reviewing published reports,14,15 the optimal concentration of LTA was determined to be 10µg/ml; no cytotoxicity was demonstrated on the basis of the MTT assay (data not shown).
Whole-cell extracts were prepared by sonication in buffer (1% Triton X-100, 20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5% glycerol, 1 mM EDTA [pH 8.0], and protease inhibitor cocktail). After centrifugation (12,000 × g, 15 min at 4℃), the protein concentration of the supernatant was measured by using a Coomassie plus protein assay reagent kit (Pierce, Rockford, IL, USA). The cell extract (30µg) in SDS sample buffer (50 mM Tris-HCl [pH 6.8], 10% glycerol, 2% SDS, 0.1% bromophenol blue, and 5% β-mercaptoethanol) was separated by 10% SDS-PAGE and transferred onto a PVDF membrane (0.45µm; Millipore, Billerica, MA, USA). After incubation with blocking solution (5% skim milk in Tris-buffered saline with 0.1% Tween-20 [TBS-T]) for 1 h at room temperature, membranebound proteins were probed overnight with anti-COX-2 (ab15191, 1 : 1,000; Abcam, Cambridgeshire, UK) and mouse monoclonal β-actin antibodies (ab-6276, 1 : 5,000; Abcam), followed by incubation with HRPconjugated goat anti-rabbit IgG (1 : 5,000) or antimouse IgG (1 : 5,000). Antibody-bound protein bands were visualized by enhanced chemiluminescence detection. Densitometric analysis for COX-2 expression was based on three independent experiments.
Total RNA was isolated by using an RNeasy mini-kit (Qiagen, Fremont, CA, USA) according to the manufacturer's instructions. cDNA was generated from 1µg of total RNA by using the Omniscript RT kit (Qiagen). For semi-quantitative PCR, a PCR pre-mixture kit (ELPIS, Taejeon, Korea) was used. PCR reactions for TNF-α and GAPDH were performed by using methods described before.12 PCR products were analyzed using 1.5% agarose gel electrophoresis, stained with SYBR safe gel stain buffer (Invitrogen, Carlsbad, CA, USA), and visualized by luminescence (LAS 3000, Fujifilm, Tokyo, Japan). Triplicate experiments were performed for the same condition, and the relative intensity for positive bands was analyzed by use of a densitometer.
Immunocytochemistry for confocal microscopy was performed on vimentin. DFs were seeded in each well of the slide chamber at a density of 3×103 cells per well. After washing with PBS, cells were fixed in 4% paraformaldehyde in PBS for 10 min and were then permeabilized with PBS containing 0.5% Triton X-100 (PBS-T) for 15 min. The cells were incubated with PBS-T containing 1% BSA for 1 h and stained with anti-vimentin antibody (M0725, 1 : 300; DakoCytomation, Carpenteria, CA, USA) overnight at 4℃, followed by incubation with Alexa Fluor 488 chicken anti-mouse IgG (A21200, 1 : 500; Invitrogen) for 1 h. Images were visualized by confocal microscopy with a 20X objective on a laser scanning microscope (LSM 510; Carl Zeiss, Jena, Germany) and were analyzed by using the LSM 5 browser imaging software.
As a preliminary experiment, we evaluated the optimal concentration of an H. cordata extract. As shown by the MTT assay, the viability of the DFs was not significantly affected by H. cordata at doses of 1~100µg/ml (Fig. 2A). However, COX-2 protein expression was stimulated by the highest dose of H. cordata at 50µg/ml (Fig. 2B), implying a toxic response of DFs to H. cordata. There was no effect of H. cordata treatment on TNF-α expression (Fig. 2C). Thus, the next experiments with H. cordata were performed at concentrations up to 20µg/ml. Among the pro-inflammatory molecules involved in the pathogenesis of AD inflammation, TNF-α was shown to be a major cytokine in inducing AD inflammation.6 In DFs treated with LTA (10µg/ml) for 24 h, the level of TNF-α mRNA based on RT-PCR was up-regulated in a dose-dependent manner (Fig. 3A). As shown by Western blot analysis, the level of COX-2 protein was up-regulated with a positive correlation between the LTA dose and COX-2 level (Fig. 3B).
We next evaluated whether H. cordata had anti-inflammatory activity in LTA-induced inflammation in DFs. When DFs were treated with H. cordata extract at doses of 20µg/ml for 24 h, the level of LTA (10µg/ml)-induced TNF-α mRNA was reduced to approximately 40% of LTA-treated control samples (p<0.05, n=3) (Fig. 4). Under the same conditions, the level of LTA-induced COX-2 protein was reduced to 52% of that in LTA-treated control samples (p<0.05, n=3) (Fig. 5).
From the results that LTA could induce TNF-α mRNA expression, we evaluated whether H. cordata had anti-inflammatory activity in TNF-α-treated DFs. When DFs were treated with H. cordata extract at doses of 20µg/ml for 24 h, TNF-α-induced COX-2 expression was reduced to 65% of that in TNF-α-treated control samples (p<0.05, n=3) (Fig. 6).
H. cordata is a native herbaceous plant in many Asian countries. H. cordata has been used to treat various infectious conditions owing to its anti-viral activ ity against herpes virus type 1, influenza virus, and human immunodeficiency16 and its antibacterial activity against S. aureus, Sarcina ureae,17 Propiniobacterium acnes (P. acnes),18 and Salmonella typhimurium.19 H. cordata also has other biological activities, such as anti-inflammatory,19 anti-cancer,20 and anti-oxidant activities.21 Some components of H. cordata, including methyl nonyl ketone, caryophyllene, bornyl acetate, α-pinene, β-pinene, and limonene, have been reported to have antibacterial properties against the Gram-positive bacteria S. aureus and Sarcina ureae.17 With respect to antioxidant activity, other components of H. cordata, including quercitrin, quercetin-3-O-β-D-galactopyranose, and protocatechuic acid, were isolated from H. cordata extract.21 AD is a chronically relapsing skin disease manifesting with recurrent attacks of eczematous inflammation in the skin. AD develops from complex interactions between genetic alteration and environmental agents. Among several proposed environmental factors, S. aureus is extensively studied in relation to the pathogenesis of AD, because the microorganism was revealed to be a major pathogen for inducing inflammatory and immunologic responses in the skin of AD patients. The altered skin structure or barrier function and a deficiency of antimicrobial peptides favor colonization of S. aureus in the skin of patients with AD, because their endotoxins with superantigenic activity stimulate the activation of T cells or other cells involved in immunologic responses.22 Taken together, H. cordata might be a candidate therapeutic agent to treat AD by controlling inflammatory or oxidative responses as well as S. aureus infections in the skin of patients with AD.
The results of this study showed that H. cordata has a strong anti-inflammatory activity against S. aureus LTA-induced inflammatory responses, partly through inhibiting TNF-α expression in DFs. In this study, H. cordata extract did not affect the expression of COX-2 or TNF-α at doses of 10~20µg/ml, but H. cordata extract at 50µg/ml up-regulated COX-2 expression, suggesting a toxic response of DFs at the highest dose of H. cordata. Among the cellular components of skin, KCs are known to be the major cells that produce TNF-α, and DFs serve as a target of TNF-α that is secreted from KCs or inflammatory cells, including lymphocytes, monocytes, mast cells, and macrophages.23 S. aureus LTA can efficiently stimulate human T cells and monocytes to produce TNF-α via Toll-like receptor-2.24 TNF-α has myriad pro-inflammatory effects on skin inflammation, but TNF-α is barely detectable from KCs or DFs of the skin under normal conditions. However, ultraviolet (UV)-B irradiation induces TNF-α from KCs and DFs,10,11 implying that UV radiation or proinflammatory stimuli induce the production of TNF-α from both cells. In agreement with our previous report that P. acnes induced TNF-α production from DFs,12 this study showed that LTA also stimulates the production of TNF-α from DFs. Because the inflammatory reaction of AD takes place in the dermis rather than the epidermis, our results suggest that DFs are not only a target of TNF-α that is produced by other cells (KCs and inflammatory cells) but also a source for TNF-α expression by LTA stimulation. When DFs were treated with TNF-α, H. cordata had an inhibitory effect against TNF-α-induced COX-2 expression.
Among the three isoforms of COX, COX-2 is widely studied owing to its diverse physiologic and pathologic processes, including inflammation. COX-2 is also induced in response to inflammatory stimuli, such as UV radiation, lipopolysaccharide (LPS), and 12-O-tetradecanoylphorbol 13-acetate (TPA) or phorbol ester in the skin.13,25-27 In this study, we used TNF-α and COX-2 as inflammatory markers of DFs.
The cytokine production by LTA is similar to that induced by whole bacteria.28 LTA from S. aureus can promote the induction of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 from monocytes or macrophages,29 which was also induced by whole bacteria. In this study, we found that LTA induces an inflammatory response by stimulating TNF-α production as well as COX-2 in DFs. Taken together, our results showing that H. cordata has inhibitory activity against LTA-induced inflammation in DFs suggest that H. cordata might be a novel anti-inflammatory agent for relieving AD inflammation. The present study supports the traditional medicine practice in Korea in which H. cordata is used as an anti-inflammatory agent to relieve inflammatory conditions, such as acne and AD. Further studies should be performed to clarify which components of H. cordata have anti-inflammatory activity in LTA-induced inflammation in DFs. Our in vitro results should be validated by in vivo experiments in animals and humans in the future.
Figures and Tables
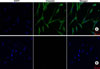 | Fig. 1Immunocytochemical staining for vimentin in DFs. (A) Confocal laser scanning microscopy demonstrated positive fluorescence signals for vimentin (green). Nuclei were stained with DAPI (blue). (B) Negative controls stained only with secondary antibody. Scale bar: 50µm. |
 | Fig. 2Cell viability assay and levels of imflammatory markers to determine optimal concentrations of H. cordata and LTA in DFs. (A) DFs were treated with H. cordata water extracts at different concentrations (1µg/ml to 200µg/ml), and cell viability was measured with an MTT assay kit. The cell viability of control samples, which were not treated with H. cordata extract, was designated as 100%. The data represent the mean viability values of six samples that were treated with the same concentration of H. cordata extract. (B) Western blot analysis for the level of COX-2 expression in DFs, which were treated with H. cordata extract at different concentrations (10~50µg/ml). C (control) indicates the level of COX-2 protein in DFs that were not treated with H. cordata extract. (C) RT-PCR for TNF-α mRNA expression was performed with samples that were harvested 24 h after H. cordata treatment (10~50µg/ml). For an internal standard, RT-PCR for the GAPDH gene was performed with each sample. *p<0.05. |
 | Fig. 3Dose-dependent expression of TNF-α and COX-2 by LTA in DFs. (A) DFs were treated with LTA at concentrations of 5, 10, and 20µg/ml, and RT-PCR for TNF-α mRNA expression was performed with samples that were harvested 24 h after LTA treatment. For an internal standard, RT-PCR for the GAPDH gene was performed with each sample. (B) Under the same conditions, protein levels of COX-2 were checked by Western blot analysis. For an internal standard, the β-actin protein level of each sample was measured. This picture is representative of three experiments. |
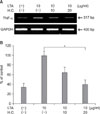 | Fig. 4Suppression of LTA-induced TNF-α expression by H. cordata extract in DFs. (A) DFs were treated with H. cordata extract (H.C.) at concentrations of 10 and 20µg/ml in the presence of LTA (10µg/ml), and RT-PCR for TNF-α mRNA expression was performed. This picture is representative of three experiments. (B) Densitometric analysis for TNF-α mRNA levels from triplicate experiments was performed, and the relative density of each sample was depicted compared to the highest level (100%) of the sample, which was only treated with LTA (10µg/ml). *p<0.05 for significant differences between the groups (n=3). |
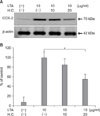 | Fig. 5Suppression of LTA-induced COX-2 expression by H. cordata extract in DFs. (A) DFs were treated with H. cordata extract (H.C.) at concentrations of 10 and 20µg/ml in the presence of LTA (10µg/ml), and Western blot analysis for COX-2 protein expression was performed. (B) Densitometric analysis for COX-2 protein levels from triplicate experiments was performed, and the relative density of each sample was depicted compared to the highest level (100%) of the sample, which was treated only with LTA (10µg/ml). *p<0.05 for significant differences between the groups (n=3). |
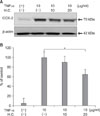 | Fig. 6Suppression of TNF-α-induced COX-2 expression by H. cordata extract in DFs. (A) DFs were treated with H. cordata extract (H.C.) at concentrations of 10 and 20µg/ml in the presence of TNF-α (10µg/ml), and Western blot analysis for COX-2 protein expression was performed. (B) Densitometric analysis for COX-2 protein levels from triplicate experiments was performed, and the relative density of each sample was depicted compared to the highest level (100%) of the sample, which was treated only with TNF-α (10µg/ml). *p<0.05 for significant differences between the groups. |
Acknowledgements
This study was supported by grant CRI08052-1 from the Chonnam National University Hospital Research Institute of Clinical Medicine.
References
1. Lu HM, Liang YZ, Yi LZ, Wu XJ. Anti-inflammatory effect of Houttuynia cordata injection. J Ethnopharmacol. 2006. 104:245–249.
2. Schnopp C, Ring J, Mempel M. The role of antibacterial therapy in atopic eczema. Expert Opin Pharmacother. 2010. 11:929–936.

3. Ginsburg I. Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis. 2002. 2:171–179.

4. Xia G, Kohler T, Peschel A. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int J Med Microbiol. 2010. 300:148–154.

5. Matsui K, Nishikawa A. Lipoteichoic acid from Staphylococcus aureus enhances allergen-specific immunoglobulin E production in mice. Clin Exp Allergy. 2003. 33:842–848.

6. Numerof RP, Asadullah K. Cytokine and anti-cytokine therapies for psoriasis and atopic dermatitis. BioDrugs. 2006. 20:93–103.

7. Albanesi C. Keratinocyte in allergic skin diseases. Curr Opin Allergy Clin Immunol. 2010. 10:452–456.
8. Chaly YV, Selvan RS, Fegeding KV, Kolesnikova TS, Voitenok NN. Expression of IL-8 gene in human monocytes and lymphocytes: differential regulation by TNF and IL-1. Cytokine. 2000. 12:636–643.

9. Aufiero B, Guo M, Young C, Duanmu Z, Talwar H, Lee HK, et al. Staphylococcus aureus induces the expression of tumor necrosis factor-alpha in primary human keratinocytes. Int J Dermatol. 2007. 46:687–694.

10. Bashir MM, Sharma MR, Werth VP. TNF-alpha production in the skin. Arch Dermatol Res. 2009. 301:87–91.
11. De Kossodo S, Cruz PD Jr, Dougherty I, Thompson P, Silva-Valdez M, Beutler B. Expression of the tumor necrosis factor gene by dermal fibroblasts in response to ultraviolet irradiation or lipopolysaccharide. J Invest Dermatol. 1995. 104:318–322.

12. Choi JY, Piao MS, Lee JB, Oh JS, Kim IG, Lee SC. Propionibacterium acnes stimulates pro-matrix metalloproteinase-2 expression through tumor necrosis factor-alpha in human dermal fibroblasts. J Invest Dermatol. 2008. 128:846–854.

13. Lee JL, Mukhtar H, Bickers DR, Kopelovich L, Athar M. Cyclooxygenases in the skin: pharmacological and toxicological implications. Toxicol Appl Pharmacol. 2003. 192:294–306.

14. Baroni A, Perfetto B, Ruocco E, Rossano F. Lipoteichoic acid and protein-A from Staphylococcus aureus stimulate release of hepatocyte growth factor (HGF) by human dermal fibroblasts. Arch Dermatol Res. 1998. 290:211–214.

15. Perfetto B, Donnarumma G, Criscuolo D, Paoletti I, Grimaldi E, Tufano MA, et al. Bacterial components induce cytokine and intercellular adhesion molecules-1 and activate transcription factors in dermal fibroblasts. Res Microbiol. 2003. 154:337–344.

16. Hayashi K, Kamiya M, Hayashi T. Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus, and HIV. Planta Med. 1995. 61:237–241.

17. Lu H, Wu X, Liang Y, Zhang J. Variation in chemical composition and antibacterial activities of essential oils from two species of Houttuynia Thumb. Chem Pharm Bull (Tokyo). 2006. 54:936–940.

18. Chomnawang MT, Surassmo S, Nukoolkarn VS, Gritsanapan W. Effect of Garcinia mangostana on inflammation caused by Propionibacterium acnes. Fitoterapia. 2007. 78:401–408.

19. Kim GS, Kim DH, Lim JJ, Lee JJ, Han DY, Lee WM, et al. Biological and antibacterial activities of the natural herb Houttuynia cordata water extract against the intracellular bacterial pathogen salmonella within the RAW 264.7 macrophage. Biol Pharm Bull. 2008. 31:2012–2017.

20. Kim SK, Ryu SY, No J, Choi SU, Kim YS. Cytotoxic alkaloids from Houttuynia cordata. Arch Pharm Res. 2001. 24:518–521.
21. Chou SC, Su CR, Ku YC, Wu TS. The constituents and their bioactivities of Houttuynia cordata. Chem Pharm Bull (Tokyo). 2009. 57:1227–1230.
22. Schmid-Grendelmeier P, Ballmer-Weber BK. Atopic dermatitis - current insights into path physiology and management. Ther Umsch. 2010. 67:175–185.
23. Graham GM, Farrar MD, Cruse-Sawyer JE, Holland KT, Ingham E. Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br J Dermatol. 2004. 150:421–428.

24. Ellingsen E, Morath S, Flo T, Schromm A, Hartung T, Thiemermann C, et al. Induction of cytokine production in human T cells and monocytes by highly purified lipoteichoic acid: involvement of Toll-like receptors and CD14. Med Sci Monit. 2002. 8:BR149–BR156.
25. Fujii E, Irie K, Ogawa A, Ohba K, Muraki T. Role of nitric oxide and prostaglandins in lipopolysaccharide-induced increase in vascular permeability in mouse skin. Eur J Pharmacol. 1996. 297:257–263.

26. Wang HQ, Smart RC. Overexpression of protein kinase C-alpha in the epidermis of transgenic mice results in striking alterations in phorbol ester-induced inflammation and COX-2, MIP-2 and TNF-alpha expression but not tumor promotion. J Cell Sci. 1999. 112:3497–3506.

27. Athar M, An KP, Morel KD, Kim AL, Aszterbaum M, Longley J, et al. Ultraviolet B (UVB)-induced cox-2 expression in murine skin: an immunohistochemical study. Biochem Biophys Res Commun. 2001. 280:1042–1047.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download