Introduction
Spontaneous coronary artery dissection (SCAD) is a rare but important cause of acute coronary syndrome. Spontaneous coronary artery dissection is the separation of the layers of the arterial wall, making a false lumen. The true lumen can be compromised by the expansion of hematoma in the false lumen, which causes acute coronary syndrome.1 The first case report of SCAD was in 1931.2 Many cases have been diagnosed by autopsy, because SCAD presented as sudden death. In one series, 75% of cases occurred in women, half of which were associated with the postpartum state.3
The overall incidence of SCAD in coronary artery angiography ranges from 0.28% to 1.1%.4 Seventy percent of SCAD occurs in women, 30% of which is associated with a postpartum state.3 The most common involved location of the coronary artery is the left anterior descending artery, which accounts for over 60%. There are a few reports about SCAD associated with vasospasm.4
We report a case of SCAD that developed in both the culprit and non-culprit arteries of acute myocardial infarction associated with vasospasm.
Case Report
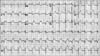
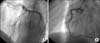
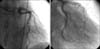
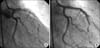
A 36-year-old man presented with continuous chest pain for 1 hour. He had been treated in the nephrology department for an acute renal infarction. No thrombotic or embolic source was found on trans-esophageal echocardiography, abdominal CT angiography (CTA), or serologic tests including protein C, protein S, anti-phospholipid antibody, and anti-nuclear antibody. His initial EKG showed pathologic Q wave and ST elevation in lead II, III, and aVF (Fig. 1). The levels of cardiac enzymes were elevated: creatine kinase (CK) was 1537 U/L (35~172), CK-MB was 36.6 U/L (2.3~9.5), and troponin-I was 0.58 ng/mL (0-0.05). Emergent coronary angiography (CAG) revealed diffuse spastic narrowing of the left anterior descending coronary artery (LAD) and right coronary artery (RCA), but no significant stenosis was detected (Fig. 2). There was no regional wall motion abnormality and his ejection fraction was 69.8% on echocardiography. On the follow-up coronary angiogram, diffuse stenosis in the left main to distal LAD with spontaneous type B dissection of the proximal LAD extending to the left main and middle LAD were detected (Fig. 3). These findings were also shown by intravascular ultrasonography (IVUS) (Fig. 4A) and cardiac CT angiography (Fig. 4B, 4C). We decided to treat with medical therapy because the patient had no chest pain. Three days later, he complained of severe chest pain with a 3-fold increase in CK-MB (from 4 U/L to 14.1 U/L) and markedly elevated troponin-I (from 0.03 ng/mL to 2.20 ng/mL). His EKG showed a tall T wave in V2-4 with Q wave in II, III, and aVF. Emergency CAG revealed dissection in the left main (LM) to middle LAD and proximal LCx with poor distal flow and spasm in the distal LAD. We decided to perform percutaneous coronary intervention (PCI) for these lesions and deployed the following Cypher stents (Cordis Corp, Johnson & Johnson, Miami Lakes, FL): 3.5×18 mm for proximal LCx, 3.5×28 mm for LM to proximal LAD, 3.5×33 mm for middle LAD, and 3.0×33 mm for distal LAD. The final CAG showed a remaining small dissection in the distal LAD stent edge (Fig. 5). Apico-septal wall akinesia was detected and the patient's ejection fraction was 60% on the echocardiogram after PCI. The follow-up CAG and renal angiogram were performed 6 months later. CAG showed patent Cypher stents, and no significant stenosis in either renal artery.
Discussion
The possible conditions associated with SCAD are atherosclerosis,5 the peripartum period,4 vasculitis, polyarteritis nodosa, systemic lupus erythematosus, Marfan's syndrome, Ehlers-Danlos syndrome, hypertension, variant angina, and cocaine use.6,7 The mechanism related to atherosclerosis in SCAD is thought to be atherosclerotic plaque rupture causing disruption of the intimal-medial junction resulting in intramural hematoma formation. The mechanism of peripartum dissection, however, is related to eosinophilic infiltration in the coronary artery adventitia breaking down the medial-adventitial layers.8
In this case, the SCAD was associated with vasospasm and there was no significant atherosclerosis on IVUS. In variant angina, spasm may increase the shear stress, leading to SCAD. Eosinophils may cause vasospasm in variant angina. In an animal model, extracts from eosinophils caused strong contraction of intestinal smooth muscle.8 This supports that eosinophils can initiate the coronary spasm in humans. In relation to vasospasm, coronary dissection has been reported in cocaine use. The principal effects of cocaine are mediated by alpha-adrenergic stimulation, which causes an increase in myocardial O2 demand and a reduction in O2 supply because of coronary vasoconstriction.
In this case, we concluded that the culprit coronary artery was the left main to left anterior descending artery (LM to LAD), because apico-septal (LAD territory) wall akinesia was detected on echocardiography. On the initial CAG, spastic narrowing was detected in the left anterior descending coronary artery and the right coronary artery but no significant atherosclerosis was seen on intravascular ultrasonography (IVUS). The second CAG showed diffuse stenosis in the left main to distal LAD with spontaneous type B dissection of the proximal LAD extending to the LM and middle LAD. On the third CAG, SCAD was detected in the culprit lesion (LCX) and in the non-culprit lesion (LAD). When we diagnosed SCAD on the second CAG, the patient did not complain of chest pain or related symptoms, so we chose medical therapy. However, PCI was chosen when SCAD with AMI was detected on the third CAG. Treatment options for SCAD include medical therapy, PCI, or coronary artery bypass graft surgery. There are no randomized studies about the treatment of SCAD, but when the involved vessel is small and there is no evidence of persistent ischemia or hemodynamic instability, medical therapy can be chosen. When SCAD causes myocardial ischemia, PCI or coronary artery bypass graft surgery may be considered.9
In this case, because there was no atherosclerotic lesion, no calcified lesion, and no coronary artery anomaly, the possibility of catheter-induced dissection was very low. However, dissection of the LAD could be considered to be procedure-related because the first angiogram showed no definite dissection in the LAD. There are some reasons that we consider spontaneous dissection rather than iatrogenic origin. The first reason is that spontaneous dissection was also present in the proximal RCA, which was far from the catheter-engaged site. Dissection flow was started in the proximal LAD, which also far from the catheter-engaged site, and then extended to the left main and down to the middle LAD.
This case showed that vasospasm of the coronary artery can cause SCAD. Further randomized studies on the treatment of SCAD are needed to determine the optimal treatment.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download