Abstract
Angiogenesis and apoptosis are essential in the tumorigenesis and progression of various tumors. Cyclooxygenase-2 (COX-2) and survivin are important factors in these events; however, their roles in neuroblastomas are yet to be delineated. The aim of this study was to evaluate the expression of both COX-2 and survivin in neuroblastomas along with their relationships with tumor angiogenesis, apoptosis, and classical prognostic factors. hirty-nine various-stage neuroblastomas were evaluated for COX-2 expression, microvessel density (MVD), and survivin expression by immunohistochemical methods. The apoptotic cells were visualized by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL). Thirty-seven cases (94.9%) showed specific expression of COX-2 in the cytoplasm of the tumor cells. COX-2 expression correlated with International Neuroblastoma Staining System (INSS) stage (p=0.004), Shimada histology (p=0.006), and International Neuroblastoma Risk Group (INRG) risk factors (p=0.003). COX-2 expression was positively correlated with MVD (p=0.029), but not with apoptotic index (AI). Thirty-six cases (92.3%) showed positive survivin immunoreactivity, which was observed in the nucleus, cytoplasm, or both of tumor cells. Survivin expression correlated with patient age (p=0.019), INSS stage (p=0.001), and INRG risk factor (p<0.001). There was a significant difference in AI between the survivin-positive and the survivin-negative groups (p=0.018), and the AI was inversely correlated with survivin. The expression of COX-2 was closely correlated with the expression of survivin. COX-2 and survivin are overexpressed in neuroblastomas, which may play an important role by stimulating tumor angiogenesis and anti-apoptosis in neuroblastomas.
Neuroblastoma (NB) is the most frequently occurring solid tumor in children, with an incidence of 1~3 cases per 100,000 children aged 0~14 years. Prognosis in NB patients tends to vary greatly, and many studies have demonstrated that both clinical and molecular biological factors are correlated with outcome. Overall, the outcome for most childhood cancers has improved dramatically over the last 3~4 decades as a result of our ability to diagnose and treat children with multimodal therapies, however; survival for high-risk neuroblastoma remains less than 50%, and new approaches are needed.1
Angiogenesis is a fundamental role in the neoplastic process and is essential for the local progression and metastatic spread of solid and hematologic tumors. High vascular index is associated with poor prognosis in neuroblastoma patients and aggressive neuroblastoma growth depends on active angiogenesis.2 Folkman proposed the concept of controlling the growth of tumors by preventing their vascularization, and the development of drugs that inhibit angiogenesis in tumors including neuroblastoma has become an important goal.3
Cycloxygenases (COXs) are the rate-limiting enzymes involved in conversion of arachidonic acid to prostaglandins. Two isoforms of COX, COX-1 and COX-2, have been identified.4 Increased amount of COX-2 are found commonly in both premalignant and malignant tissues of epithelial origin in adults and has been implicated in resistance to apoptosis, promotion of cell proliferation, increased tumor invasiveness, induction of metastases and angiogenesis as well as decreased immune surveillance.5 A significant increase in COX-2 expression is also observed in neuroblastoma and its cell lines.6 And various epidemiological studies have revealed that the use of NSAIDs and a specific COX-2 inhibitor,6 induce apoptosis and inhibit both in vitro and in vivo growth of human neuroblastoma, suggesting that this pathway may be a good target for chemoprevention of neuroblastoma. Although COX-2 contributes to neovascularization and may support vasculature dependent solid tumor growth,7 the role of COX-2 alterations in the modulation of angiogenesis and apoptosis in neuroblastoma is little known and needs to be elucidated.
Apoptosis is one of the hallmarks of malignant cell transformation and tumor.8 Proteolytic enzymes such as caspases play an important role as effector molecules in apoptosis including cytotoxic therapy-induced cell death.9 The family of endogenous caspase inhibitors called 'inhibitor of apoptosis proteins' (IAPs) comprises proteins such as XIAP, cIAP1, cIAP2, survivin, and livin.10 Among them, survivin is to be associated with increased of tumor angiogenesis and inhibit the apoptosis.11 In human neuroblastomas, survivin expression was correlated with clinical outcome by inhibition of apoptosis, however, the association between survivin expression and angiogenesis in neuroblastoma has not been fully established.
Although COX-2 and survivin are involved in tumorigenesis with increased angiogenesis and inhibition of apoptosis,12,13 these events in neuroblastoma have been not reported. The aim of this study was to evaluate the expression of both COX-2 and survivin in neuroblastoma along with their relationship with tumor angiogenesis, apoptosis, and classical prognostic factors.
Patients with neuroblastoma evaluated at the Department of Pediatrics, Chonnam University Medical School, Gwangju, Korea, were diagnosed and staged according to the International Neuroblastoma Staging System (INSS). Thirty-nine paraffin-embedded samples were obtained from untreated patients with neuroblastoma. Five childhood ganglioneuromas and two samples of non-malignant adrenal medulla from children were also included. The age at diagnosis ranged from 28 days after birth to 433 months. Of the 39 cases, 28 patients were diagnosed at older than 1 year, whereas the remaining 11 were diagnosed at younger than 1 year. Of the 39 samples, 16 were tumors from patients who were stage I, II, or IVS, whereas 6 were stage III and 17 were stage IV. In neuroblastoma cases, twenty eight patients are still alive, of whom 3 cases are still under treatment, whereas 8 patients have died of the disease. The follow-up period after treatment ranged from 1 month to 12 years in 26 patients. In 9 cases of 39 samples, the status of MYCN amplification was also determined by the Southern blotting method. Regarding the histologic findings, all 39 cases were classified based on the Shimada classification. Twenty-four cases showed a favorable histology, whereas the remaining 15 cases showed an unfavorable histology. Regarding International Neuroblastoma Risk Group (INRG), all 39 cases were classified by age, stage, the status of MYCN amplification (Southern blot), if data was analyzed, and Shimada classification. Eighteen showed a not high-risk group, whereas the remaining 21 cases showed a high-risk group.
We reviewed the hematoxylin and eosin stained slides of the tumor specimens, and then selected tissue blocks from an including edge of tumor area for the formalin-fixed, paraffin-embedded samples of tumor tissue. Immunohistochemical analysis included antibodies for COX-2 (Cayman, MI, USA; dilution 1:100), survivin (Santa Cruz, CA, USA; dilution 1:800), and CD34 (Dako, Copenhagen, Denmark; dilution 1:50). All immunohistochemistry was modified from previous report.14 Briefly, representative paraffin blocks were consecutively cut at 4 micron thickness and immunohistochemical staining carried out using Sequenza rack stainer (Shandon, London, UK). Sections were deparaffinized in xylene, treated with 0.3% hydrogen peroxide in methanol for 20 min to block endogeneous peroxidase activity. For COX-2, sections were subjected to the microwave heat in citrate-phosphate buffer (pH 6.0) twice, and then incubated with antibodies at 4℃ overnight. Antigen retrieval procedure was not applied for survivin and CD34. For survivin and CD34, sections were incubated with primary antibody for 30 min at room temperature (RT). Anti-mouse immunoglobulin G (Sigma, St. Louis, MO) labeled with biotin was used as a secondary antibody for the detection of primary antibodies and was incubated for 7 minutes at RT.
The streptavidin-horseradish peroxidase (Research Genetics, USA) detection system was applied to capillary channels, followed by 10 min of incubation at 45℃. After drainage, the tissue sections were ready for chromogen reaction with 0.02% diaminobenzidine. The sections were counterstained with hematoxylin and mounted in Universal Mount (Research Genetics. USA). Negative controls were treated similarly with the exception of primary antibodies.
All immunostained slides were evaluated without any clinical data and repeated two times each. Evaluators were blinded to background information. For COX-2 assessment, reactions in smooth muscles and vascular endothelial cells, which were present in all specimens, were used as internal built-in positive controls. Protein expression of COX-2 and survivin was assessed semiquantitatively by Friedrich et al.15 COX-2 and survivin immunoreactivity was determined by summation of the values of the percentage of survivin-positive cells (0: <1%, 1: 1~25%, 2: 26~50%, 3: 51~75%, 4: >75%) and the values for survivin staining intensity (SI: 0: no staining, 1: weak staining, 2: moderate staining, 3: strong staining). Tumors having a final staining score of >2 were considered as positive, and >4 were considered as high COX-2 expression.
MVD was assessed according to the international consensus report.16 Immunostained slides were scanned at 100X magnification to identify the areas with the highest number of vessels (so called "hot spot"). Each image was captured by JVC Digital Camera system (JVC Digital Camera KY-F70B, JVC Corporation, Tokyo, Japan) attached on the Nikon microscope (Nikon E600, Tokyo, Japan) at 200× magnification. Each 200× magnification area was 0.48 mm2. Counts were performed on 5 consecutive fields by touch count in the hot spot.17 Each positive vessel was marked by red-colored '+' symbol (Fig. 1). All stained endothelial cells or cell clusters were counted as one microvessel. To clarify the CD34 staining results, image zoom-up was performed when necessary. If two or more positive foci seemed to belong to a single continuous vessel, they were counted as one microvessel. The presence of a lumen was not required. Occasional macrophages, plasma cells, and peripheral nerve bundles were stained positively for CD34. These were excluded from counts on morphological grounds. The mean of the three highest counts per tumor was taken for further analyses. The predefined cut-off value for categorical evaluation of MVD was the median MVD of the population studied.
Apoptotic cells were identified by a terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) method.18 The TUNEL method was used according to procedures included in the ApopTag Plus in situ Apoptosis Detection Kit (Intergen, Purchase, NY). Briefly, after deparaffinization and washing in phosphate-buffered saline (PBS) (50 mM sodium phosphate, pH 7.4, 200 mM NaCl), tissues were digested with proteinase K (20 µg/mL in PBS) for 15 min at room temperature and then washed with distilled water. Slides were then put into 3% H2O2 for 5 min to quench the endogenous peroxidase activity and then washed with PBS. After adding equilibration buffer for 1 min, the sections were exposed to TdT with digoxigenin-11-dUTP and dATP in a moist chamber for 60 min at 37℃. The reaction was stopped by immersing the slides into stop/wash buffer. After washing, anti-digoxigenin-peroxidase was added to the slides. Slides were washed, stained with diaminobenzidine substrate, and then counterstained with hematoxylin. To confirm the reaction specificity of the TUNEL procedure, a negative control was also run, omitting TdT from the reaction mixture. A rat intestine section with obvious apoptotic cells as the positive control. The apoptotic index (AI) was determined by counting the number of positively labeled nuclei per 1000 tumor cells. Positively labeled cells and bodies meeting the morphological characteristics of apoptosis were identified as apoptotic cells and bodies. Cells in the vicinity of necrotic areas were excluded.
Statistical analysis was performed using the SPSS software program (version 12.0 for Windows; SPSS INC., Chicago, IL). The relationship between COX-2, survivin expression and categorical variables was compared with the Chi-square test, or Fisher's exact probability test when appropriate. Continuous variables were compared with the Mann-Whitney U test. The strength of association between the COX-2, survivin, and MVD, AI was assessed by the Spearman rank correlation test. p<0.05 was considered to be significant.
The present study analyzed neuroblastomas from different biological subsets and at all clinical stages for COX-2 expression. Within the tumor, COX-2 staining pattern showed intratumoral heterogeneity. According to our scoring system, thirty-seven of 39 neuroblastoma samples (94.9%) showed specific expression of COX-2 protein in the cytoplasm of the tumor cells (Fig. 2A, and 2B). Interestingly, COX-2 expression was identified in the main cellular components of neuroblastoma including neuroblast and ganglionic cells, but not in Shcwannian stroma. No Cox-2 expression was detected in the surrounding non-malignant adrenal medulla tissues, whereas the surrounding adrenal cortex showed a positive staining for COX-2 (Fig. 2D). Two neuroblastoma sample, from a localized stage I and stage II tumor with favorable biology (sample #: 4, 9), did not express COX-2. Five ganglioneuromas were investigated and showed COX-2 staining in the tumor-derived differentiated ganglion cells, but not in the surrounding stroma (Fig. 2C). Correlation between COX-2 expression and known prognostic factors are listed in Table 1. There was statistically significant correlation between INSS stage, Shimada histology, and INRG risk factors.
Thirty-six of 39 neuroblastoma cases (92.3%) showed survivin positive immunoreactivity, and 20 cases showed high expression (more than 20% of positive cells). Survivin immunoreactivity was observed in the nucleus (Fig. 3A), cytoplasm (Fig. 3B), and both (Fig. 3C) of tumor cells, however, there was no significant difference between survivin localization and current prognostic factors in this study. Nuclear survivin expression was identified the neuroblasts, and cytoplasmic expression was noted in the ganglion. Survivin is not expressed in the Schwannian stroma. Fig. 3D shows low survivin expression in neuroblastoma. Correlation between low and high survivin expression and known prognostic factors are listed in Table 1. There was statistically significant correlation between survivin expression and age, INSS stage, and INRG risk factors.
When we correlated COX-2 expression and survivin expression, increased COX-2 was associated with increased survivin expression (r=0.334, p=0.038). Combined analysis of COX-2 and survivin expressions showed that tumors with positive expression of both factors had a statistical significance in age, INSS stage, and INRG risk factors.
All endothelial cells that were visible on the histological slides were immunostained by CD34 antibodies. The area of greatest vascular density was usually, but not always, at the periphery of the tumor. Microvessel density (MVD) ranged from 14.6 to 33.2 and had a median value of 21.7 (23.9±3.4). Acceptable results were noted for both the negative and positive controls with the TUNEL staining. A variable proportion of stained cells showed the morphologic criteria of apoptosis, namely, cell shrinkage and nuclear condensation and fragmentation (Fig. 4). Apoptotic index (AI) ranged from 2.1% to 10.3%, with a median value of 6.3 % (6.5±1.3). Between COX-2 protein expression and the MVD, we could observe a positive correlation with statistical significance (r=0.354, p=0.029). And high survivin expression tumors had significantly lower values with negative correlation for AI than low survivin expression tumors (r=-0.385, p=0.018). No significant difference was seen between COX-2 expression and AI, and between survivin and MVD. Finally, there was significant difference between high expressions of both COX-2 /survivin and MVD, but not between AI (Table 2).
The present study has investigated the COX-2 and survivin expression pattern in neuroblastoma and found that 1) COX-2 and survivin expression are closely correlated, 2) COX-2 expression directly correlate with INSS tumor stage, Shimada histology, and INRG risk factors, and increased vasculature and 3) surivin expression is correlated with patient age, INSS tumor stage, INRG risk factors, and inversely correlated with tumor apoptosis.
COX-2 has been reported to have important tumorigenic and tumor progressive properties in recent publications. These reports state the importance of COX-2 in inhibition of apoptosis, enhancement of angiogenesis, and metastatic potential.5,6,14 In this study, we find out that the neuroblastoma were expressed COX-2 and high COX-2 expression was correlated with tumor stage, INRG risk factors, and high tumor vascularity. These findings suggest that COX-2 expression is upregulated by the advancement of tumor progression of neuroblastoma through modulates the expression of potent angiogenetic factors.
Neuroblastic tumors consist of two main cell populations, neuroblastic/ganglionic cells and Schwann cells, and the ratio of these cell types varies according to tumor maturation. Immature neuroblastoma is composed of undifferentiated neuronal cells and a paucity of Schwannian stroma, whereas larger, ganglion-like cells and abundant Schwannian stroma are seen in maturing neuroblatic tumors. The favorable prognostic impact of the presence of Schwannian stroma has been emphasized in the International Neuroblastoma Pathology Classification System. It has been speculated that Schwann cells influence neuroblastic growth by secreting molecules that serve as anti-proliferative and pro-differentiation factors for neuronal cells.19 In this study, we found out that COX-2 expression in the favorable histology group is lower than unfavorable histology groups (Table 1) and COX-2 expression was correlated MVD (Table 2). These findings agreed with Schwann cells produce angiogenesis inhibitors and Schwann cells may also influence NB growth by restricting angiogenesis.
COX-2 derived prostaglandins (PGs) regulate programmed cell death and reduce the apoptotic rate via inhibition of the mitochondrial apoptotic pathway characterized by reduced cytochrome c release, attenuated caspase-9 and -3 activation and upregulation of bcl-2. Conversely, COX-inhibitors trigger both the mitochondrial and death receptor-mediated apoptotic pathways with resultant cytochrome c release.5 In the previous report,6 the selective COX-2 inhibitor Celecoxib significantly inhibited tumor growth and reduced the tumor volume in vivo via intrinsic apoptosis pathway. In this study, apoptotic index was calculated and compared with COX-2 expression. However, there was no statistical significance (p=0.137, Table 2). These result could be explain that apoptotic process in neuroblastoma may go on via COX-independent mechanisms of NSAID-mediated apoptosis.20
It has been hypothesized that genes that control apoptosis may be important in defining the course of neuroblastoma development.20 Survivin is an IAP that appears to be more selectively expressed in malignant tissue, including neuroblastoma. Interestingly, a gain in the distal arm of 17q, the locus of the Survivin gene, occurring in approximately 60% of neuroblastomas correlated with increased tumor aggression.21
To the best of our knowledge, there have been a few reports on the relation between survivin expression and apoptosis or angiogenesis of neuroblastoma. Wang et al.22 used in situ hybridization to investigate survivin expression in 26 samples diagnosed with neuroblastoma. Their results showed that survivin expression was conversely correlated with an apoptosis. In the present study, survivin protein was expressed in 92.3% (36 of 39 cases) neuroblastoma sample by immunohistochemistry and we found that AI in high survivin expressed specimens significantly decreased compared with low survivin expressed specimens. Although there was no significant difference between MVD in high survivin expressed specimens and that in low survivin expressed specimens, there was an increasing tendency of MVD. Furthermore, the survivin expression is closely correlated with INSS stage and INRG risk factors in this study. The results indicate that survivin may play a key role in tumorigenesis and progression of neuroblsatoma by its biologic activities of anti-apoptosis and angiogenesis.23 However, it should be acknowledged that this study was retrospective, and therefore has limitations, e.g., in case numbers and biases of tumor histologic subtypes, which may influence the results. In addition, the mechanisms by which survivin promote angiogenesis and inhibits cell apoptosis in neuroblastoma were not investigated in this study. Nevertheless, recent review by Fukuda et al. described similar results, and it may indirectly support our findings.23
It is noteworthy that we found survivin protein located in the cytoplasm of neuroblastoma cells, as well as in the nucleus of those. An article has been reported that different survivin distribution during cell cycle using neuroblastoma cell line.24 This finding was not completely in accord with past reports in neuroblastoma sample. Some reports described the subcellular localization of survivin. In this context, it useful to review that survivin exists in two subcellular pools: 80% cytosolic and 20% nuclear.25 And at least three different splice variants of Survivin (ie, Survivin, Survivin delta Ex 3, and Survivin-2B) have been identified and have different subcellular localizations. Survivin and Survivin 2B isoforms localise predominantly to the cytoplasm, whereas the delta Ex 3 isoform is preferentially localized in the nucleus.25 Nuclear survivin may regulate cell proliferation whereas cytoplasmic survivin may be involved in cell survival but not cell proliferation, and increased survivin as seen in malignancies may therefore participate in aberrant mitotic activity leading to aneuploidy in tumors.26 Here in, we reported the different subcellular distributions of survivin in neuroblastoma. Nuclear survivin expression was mainly noted in the undifferentiated neuroblasts or Stroma-poor neuroblastoma group, and cytoplasmic survivin expression was noted in the differentiated ganglionic cells. Although there was no significant difference between subcellular survivin localization and known prognostic factor, further evaluation should be performed in large scale cases to clarify the role of the accumulation of survivin protein in cytoplasm as well as in the nucleus in neuroblastoma may be understood properly.
Finally, there was little information between COX-2 and survivin in the tumorigenesis.27 This study showed the upregulation and positive correlations of COX-2 and survivin in neuroblastoma. Although our data suggest such a relationship in neuroblastoma, the cause cannot be established. However, some suggestions can be made from the findings of our study and other available data. Angiogenic factors such as VEGF induce survivin expression in endothelial cells during vascular remodeling and angiogenesis.27 VEGF up-regulate survivin via the PI3K/Akt cell signaling pathway in human neuroblastoma cells. As we know, COX-2 is potent VEGF stimulator,7 activate PI3K/Akt signaling pathway in neuroblastoma,28 and modulated the mitochondrial apoptotic pathway.5 And constitutive overexpression of COX-2 leads to stabilization of the inhibitor of apoptosis protein survivin resulting in the elevated apoptosis resistance of COX-2-overexpressing cells.29 Genetic or pharmacologic suppression of COX-2 activity increased proteasomal degradation of survivin and cellular response to apoptosis induction. However, in this study, COX-2/survivin co-expression group is correlated with higher tumor angiogenesis rather than apoptosis, and suggest that COX-2 and survivin play a pivotal role in the tumorigenesis of neuroblastoma via regulation of angiogenesis and apoptosis.
In conclusion, this study has shown that COX-2 and survivin are overexpressed in neuroblastoma and positively correlated with INSS tumor stage, and INRG risk factors. COX-2 dependent expression of survivin is critical for tumor angiogenesis in neuroblastoma. These molecular pathways should be investigated further using neuroblastoma models.
Figures and Tables
 | Fig. 2Immunohistochemiscal staining of COX-2. Specific COX-2 expression in the cytoplasm of tumor cells in neuroblastoma, Schwannian stroma-poor (A), differentiating neuroblasts with nuclear and cytoplasmic differentiation (B), tumor-derived differentiated ganglion in ganglioneuroma (C), and adrenal cortex in non-malignant adrenal tissue (D) were noted (COX-2 immunostaining, original maginification, ×200). |
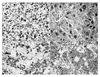 | Fig. 3Immunohistochemical staining of Survivin in neuroblastoma. Different distributions of survivin expression were noted in nucleus of Schwannian stroma-poor, undifferentiated subtype (A), cytoplasm in neuroblasts with cytoplasmic differentiation of Schwannian stroma-poor, differentiating subtype (B), and both areas in neuroblasts of Schwannian stroma-poor, poorly differentiated subtype (C). In the Schwannian stroma-poor, poorly differentiated subtype case, low survivin expression in nucleus are also noted (D) (Survivin Immunohistochemistry, ×200). |
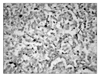 | Fig. 4Apoptotic cells (arrows) are shown by the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling technique (TUNEL assay, original maginification, ×200). |
Acknowledgements
This study was financially supported by Chonnam National University, 2005 and Research Institute of Medical Sciences, Chonnam National University, 2006.
References
2. Ribatti D, Raffaghello L, Pastorino F, Nico B, Brignole C, Vacca A, et al. In vivo angiogenic activity of neuroblastoma correlates with MYCN oncogene overexpression. Int J Cancer. 2002. 102:351–354.

4. Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996. 271:33157–33160.

5. Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003. 24:96–102.

6. Johnsen JI, Lindskog M, Ponthan F, Pettersen I, Elfman L, Orrego A, et al. Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Res. 2004. 64:7210–7215.

7. Fosslien E. Review: molecular pathology of cyclooxygenase-2 in cancer-induced angiogenesis. Ann Clin Lab Sci. 2001. 31:325–348.
10. Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998. 58:5315–5320.
11. Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008. 14:5000–5005.

12. Li S, Tong Q, Zhang W, Wang Q, Chen Z, Wu Q. Mechanism of growth inhibitory effects of cyclooxygenase-2 inhibitor-NS398 on cancer cells. Cancer Invest. 2008. 26:333–337.

13. Luo SM, Liu RD, Li WR, Hou JH. Survivin and COX-2 expressions in giant cell tumor of bone and their relation to the prognosis. Nan Fang Yi Ke Da Xue Xue Bao. 2009. 29:156–159.
14. Kim HS, Youm HR, Lee JS, Min KW, Chung JH, Park CS. Correlation between cyclooxygenase-2 and tumor angiogenesis in non-small cell lung cancer. Lung Cancer. 2003. 42:163–170.

15. Friedrich M, Villena-Heinsen C, Reitnauer K, Schmidt W, Tilgen W, Reichrath J. Malignancies of the uterine corpus and immunoreactivity score of the DNA "mismatch-repair" enzyme human Mut-S-homologon-2. J Histochem Cytochem. 1999. 47:113–118.

16. Vermeulen PB, Gasparini G, Fox SB, Toi M, Martin L, McCulloch P, et al. Quantification of angiogenesis in solid human tumours: an international consensus on the methodology and criteria of evaluation. Eur J Cancer. 1996. 32:2474–2484.

17. Kim HS, Kang HS, Messam CA, Min KW, Park CS. Comparative evaluation of angiogenesis in gastric adenocarcinoma by nestin and CD34. Appl Immunohistochem Mol Morphol. 2002. 10:121–127.

18. Lee JS, Kim HS, Jung JJ, Lee MC, Park CS. Angiogenesis, cell proliferation and apoptosis in progression of cervical neoplasia. Anal Quant Cytol Histol. 2002. 24:103–113.
19. Ambros IM, Zellner A, Roald B, Amann G, Ladenstein R, Printz D, et al. Role of ploidy, chromosome 1p, and Schwann cells in the maturation of neuroblastoma. N Engl J Med. 1996. 334:1505–1511.

20. Pritchard J, Hickman JA. Why does stage 4s neuroblastoma regress spontaneously? Lancet. 1994. 344:869–870.

21. Caron H. Allelic loss of chromosome 1 and additional chromosome 17 material are both unfavourable prognostic markers in neuroblastoma. Med Pediatr Oncol. 1995. 24:215–221.

22. Wang JX, Zheng S. Caspase-3 and survivin expression in pediatric neuroblastoma and their roles in apoptosis. Chin Med J (Engl). 2004. 117:1821–1824.
23. Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther. 2006. 5:1087–1098.

24. Chandele A, Prasad V, Jagtap JC, Shukla R, Shastry PR. Upregulation of survivin in G2/M cells and inhibition of caspase 9 activity enhances resistance in staurosporine-induced apoptosis. Neoplasia. 2004. 6:29–40.

25. Mahotka C, Liebmann J, Wenzel M, Suschek CV, Schmitt M, Gabbert HE, et al. Differential subcellular localization of functionally divergent survivin splice variants. Cell Death Differ. 2002. 9:1334–1342.

26. Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998. 396:580–584.

27. Mesri M, Morales-Ruiz M, Ackermann EJ, Bennett CF, Pober JS, Sessa WC, et al. Suppression of vascular endothelial growth factor-mediated endothelial cell protection by survivin targeting. Am J Pathol. 2001. 158:1757–1765.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download