Abstract
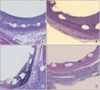
Stent thrombosis and in-stent restenosis are still major limitations of coronary stenting. Here, we report on a new type of drug-eluting stent (DES) aimed to prevent restenosis and thrombosis. The DES was prepared by coating a bare metal stent with echinomycin (an anti-proliferative drug) and hydrophobic heparin. The echinomycin-hydrophobic heparin double-coating stents were compared with control stents in a porcine coronary stent restenosis model. Stent overdilation injury (stent : artery=1.1 : 1.0) was compared in porcine coronary arteries between 4 groups: bare stent group (Group I, n=3), 1% echinomycin-heparin coating stent group (Group II, n=3), 5% echinomycin-heparin coating stent group (Group III, n=3), and 10% echinomycin-heparin coating stent group (Group IV, n=3). Follow-up quantitative coronary angiography was performed 4 weeks after the stents were inserted. The histopathologic assessments of the stented porcine coronary arteries were compared between the 4 groups. The neointimal areas of the stented arteries were 4.3±1.3 mm2 in Group I, 5.7±1.3 mm2 in Group II, 3.7±1.0 mm2 in Group III, and 2.7±1.1 mm2 in Group IV; the neointimal area was significantly larger in Group II than in the other groups (p<0.001). The histopathologic areas of stenosis were 61.4±18.1% in Group I, 79.8±24.0% in Group II, 52.0±18.5% in Group III, and 30.9±14.6% in Group IV; the histopathologic area was significantly smaller in Group IV than in the other groups (p<0.001). In conclusion, echinomycin-hydrophobic heparin double-coating stents effectively inhibit neointimal proliferation at a relatively high level.
Figures and Tables
References
1. Holmes DR Jr, Leon MB, Moses JW, Popma JJ, Cutlip D, Fitzgerald PJ, et al. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation. 2004. 109:634–640.

2. Salam AM, al Suwaidi J, Holmes DR Jr. Drug-eluting coronary stents. Curr Probl Cardiol. 2006. 31:8–119.

3. Leon MB, Bakhai A. Drug-eluting stents and glycoprotein IIb/IIIa inhibitors: combination therapy for the future. Am Heart J. 2003. 146(4):Suppl. S13–S17.

4. Mintz GS, Tinana A, Hong MK, Lee CW, Kim JJ, Fearnot NE, et al. Impact of preinterventional arterial remodeling on neointimal hyperplasia after implantation of (non-polymer-encapsulated) paclitaxel-coated stents: a serial volumetric intravascular ultrasound analysis from the ASian Paclitaxel-Eluting Stent Clinical Trial (ASPECT). Circulation. 2003. 108:1295–1298.

5. Nebeker JR, Virmani R, Bennett CL, Hoffman JM, Samore MH, Alvarez J, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol. 2006. 47:175–181.
6. Virmani R, Farb A, Guagliumi G, Kolodgie FD. Drug-eluting stents: caution and concerns for long-term outcome. Coron Artery Dis. 2004. 15:313–318.

7. Azarbal B, Currier JW. Allergic reactions after the implantation of drug-eluting stents: is it the pill or the polymer? J Am Coll Cardiol. 2006. 47:182–183.
8. Serruys PW, van Hout B, Bonnier H, Legrand V, Garcia E, Macaya C, et al. Randomised comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary artery disease (Benestent II). Lancet. 1998. 352:673–681.

9. de Scheerder I, Wang K, Wilczek K, Meuleman D, vanAmsterdam R, Vogel G, et al. Experimental study of thrombogenicity and foreign body reaction induced by heparin-coated coronary stents. Circulation. 1997. 95:1549–1553.

10. Ragosta M, Karve M, Brezynski D, Humphries J, Sanders JM, Sarembock IJ, et al. Effectiveness of heparin in preventing thrombin generation and thrombin activity in patients undergoing coronary intervention. Am Heart J. 1999. 137:250–257.

11. Ahn YK, Jeong MH, Kim JW, Kim SH, Cho JH, Cho JG, et al. Preventive effects of the heparin-coated stent on restenosis in the porcine model. Catheter Cardiovasc Interv. 1999. 48:324–330.

12. Park HW, Jeong MH, Jang YS, Kim YR, Kim W, Park WS, et al. The effects of the heparin-coated maximum arterial re-creation(MAC) stent on porcine coronary stent restenosis. Korean Circ J. 1999. 29:498–506.

13. Park HW, Jeong MH, Park OY, Kim IS, Choi MJ, Lee SH, et al. The long-term clinical effects of heparin-coated coronary stent. Korean Circ J. 2002. 32:773–780.

14. Morice MC, Serruy PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al. A randomized comparison of a sirolimus-eluting stent with standard stent for coronary revascularization. N Engl J Med. 2002. 346:1773–1780.

15. Abizaid A, Costa MA, Blanchard D, Albertal M, Eltchaninoff H, Guagliumi G, et al. Sirolimus-eluting stents inhibit neointimal hyperplasia in diabetic patients: insights from the RAVEL Trial. Eur Heart J. 2004. 25:107–112.

16. Kang WC, Han SH, Ahn TH, Son MS, Son JW, Shin EK. The long-term clinical outcomes of primary PTCA with heparincoated stent in acute myocardial infarction. Korean Circ J. 2004. 34:540–547.

17. Park HW, Jeong MH, Park OY, Kim IS, Choi MJ, Lee SH, et al. The Long-term clinical effects of heparin-coated coronary stent. Korean Circ J. 2002. 32:773–780.

18. Kim W, Jeong MH, Lim SY, Lee SR, Kim KH, Sohn IS, et al. The first clinical trial of antioxidant, carvedilol-eluting stent in coronary artery diseases. Korean Circ J. 2006. 36:115–120.

19. Hong YJ, Jeong MH, Kim W, Lim SY, Lee SH, Hong SN, et al. Effect of abciximab-coated stent on in-stent intimal hyperplasia in human coronary arteries. Am J Cardiol. 2004. 94:1050–1054.

20. Hong YJ, Jeong MH, Kim W, Lim SY, Hong SN, Lee SH, et al. The effects of abciximab(ReoPro®)-coated stents on extracellular matrix synthesis and apoptosis. Korean Circ J. 2005. 35:290–301.

21. Kim W, Jeong MH, Kim KH, Sohn IS, Hong YJ, Park HW, et al. The clinical results of a platelet glycoprotein IIb/IIIa receptor blocker (Abciximab: ReoPro®)-coated stent in acute myocardial infarction. J Am Coll Cardiol. 2006. 47:933–938.

22. Lim SY, Bae EH, Jeong MH, Kim JH, Kim JH, Joo SY, et al. The Effect of Alpha Lipoic Acid in Porcine In-Stent Restenosis Model. Korean Circulation J. 2006. 36:495–502.
23. Serruys PW, van Hout B, Bonnier H, Legrand V, Garcia E, Macaya C, et al. Randomised comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary artery disease (Benestent II). Lancet. 1998. 352:673–681.

24. Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005. 65:9047–9055.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download