Abstract
A 69-year-old male visited our medical center with hematemesis. Gastrofibroscopy revealed a 4.5 cm sized fungating mass on anterior wall of gastric lower body. Biopsy specimens showed a carcinoma of neuroendocrine components with strong positive for synaptophysin stain. Because he had a metastatic neuroendocrine carcinoma with multiple metastasis of liver, we treated him with chemotherapy of etoposide and cisplatin. The primary lesion showed nearly complete response after 6th cycles of chemotherapy, however it was regrowed with chemoresistance and mutifocal lesion in stomach and liver. Endoscopic biopsy on same previous lesion revealed a poorly differentiated tubular adenocarcinoma with negative for synaptophysin. After conversion to another tumor type, the treatment outcome was progressed in spite of salvage chemotherapy for gastric adenocarcinoma. He died 17 months after diagnosis. The immunohistological change of same mass after chemotherapy suggests a possibility of other course of differentiation from common pleuripotent cells of adenocarcinoma and neuroendocrine carcinoma after chemotherapy.
Figures and Tables
Fig. 1
Endoscopic finding. (A) Anterior wall side of gastric lower body shows a 4-5 cm sized large protruding submucosal mass-like lesion with exudates and exposed vessels at diagnosis. (B) The mass of same lesion shows dramatic regression after 3rd EP chemotherapy. (C) Anterior wall side of gastric upper body shows a new ulcerative lesion after 9th EP chemotherapy. (D) The same lesion shows a 7 cm sized aggressive mass with multiple ulceration and exudate at 15 momths from diagnosis.
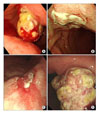
Fig. 2
The histology at diagnosis revealed relatively smaller tumor cells with granular cytoplasm, centrally located
round nuclei (A, hematoxylin&eosin staining, ×100) and strong positive for synaptophysin (B, ×400). After 9th EP chemotherapy, it showed a neoplasm with a poorly differentiated conventional glandular carcinoma (C, hematoxylin&eosin staining, ×100) and negative for synaptophysin (D, ×400).
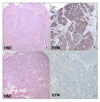
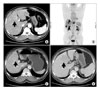
Fig. 3
(A) CT finding shows a 3.4 cm sized biggest metastatic lesion (arrow) located in Rt. lobe of liver at diagnosis. (B) PET finding shows four metastatic lesions of liver in both lobe of liver at diagnosis. (C) It shows almost regression of the same biggest lesion (arrow) in liver during 1st line EP regimen. (D) It shows a progressed lesion with another feature in same location of previously shown mass (arrow) at 15 months from diagnosis.
References
1. Gilligan CJ, Lawton GP, Tang LH, West AB, Modlin IM. Gastric carcinoid tumors: the biology and therapy of an enigmatic and controversial lesion. Am J Gastroenterol. 1995. 90:338–352.
2. Plöckinger U. Diagnosis and treatment of gastric neuroendocrine tumors. Wien Klin Wochenschr. 2007. 119:570–572.

3. Godwin JD 2nd. Carcinoid tumors. An analysis of 2,837 cases. Cancer. 1975. 36:560–569.
4. Choi YC, Roe IH, Lee JH, Park SS, Lee DH. Synchronous association of carcinoid tumor with adenocarcinoma of the stomach. Korean J Gastroenterol. 1988. 20:421–425.
5. Yamashina M, Flinner RA. Concurrent occurrence of adenocarcinoma and carcinoid tumor in the stomach: a composite tumor or collision tumors? Am J Clin Pathol. 1985. 83:233–236.

6. Kim YE, Park KC, Kwon JG. A case of double primary cancer-early gastric adenocarcinoma associated with adenocarcinama and carcinoid. Korean J Gastroenterol. 2003. 42:533–538.
7. Shin DG, Kim BS, Jang SJ, Choi WY, Kim YJ, Yook JH, et al. Neuroendocrine carcinoma of the stomach: a clinicopathologic study of 18 cases. J Korean Gastric Cancer Assoc. 2003. 3:191–194.

8. Ronellenfitsch U, Ströbel P, Schwarzbach MH, Staiger WI, Gragert D, Kähler G. A composite adenoendocrine carcinoma of the stomach arising from a neuroendocrine tumor. J Gastrointest Surg. 2007. 11:1573–1575.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download