Abstract
This study reviews evidence for the use of acetyl-choline esterase inhibitors (AChEIs) in treating Alzheimer's patients for the past decades. Even though large number of clinical trials have been conducted to prove the efficacy of these drugs in various clinical situations, questions remain to be answered due to the use of heterogeneous subject population, trial designs, and measurement tools in these studies. Many drugs with unproven clinical benefits, including vitamins, ginko biloba extracts, anti-inflammatory agents, estrogen hormone, and statins, are commonly prescribed in real-world settings for dementia patients. Despite the lack of clinical benefits statistically proven by clinical trials or metaanalyses, anecdotal dramatic improvements in some patients may foster such practices. A further look into why some patients benefit from these medications, while other don't, may shed light on future individually tailored medicine for dementia patients. This study provides a brief review of currently existing immuno-therapeutics in the hope that we can learn from the failures of the amyloid-based active and passive immunization. Issues that we need to address for the successful development of new anti-AD drugs include : 1) the brain pathology precedes clinical symptoms by several decades, 2) we need biological markers that reliably reflect cognitive or functional impairment of AD patients, and 3) we need more detailed and plausible explanations for our brain immune responses and neurodegenerative changes.
Go to : 
REFERENCES
1). Bartus RT, Dean RL 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982; 217:408–414.

2). Davis KL, Thal LJ, Gamzu ER, Davis CS, Woolson RF, Gracon SI, et al. The Tacrine Collaborative Study Group. A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer's disease. N Engl J Med. 1992; 327:1253–1259.

3). Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson's disease dementia and cognitive impairment in Parkinson's disease. Cochrane Database Syst Rev. 2012. CD006504.

4). Wang HF, Yu JT, Tang SW, Jiang T, Tan CC, Meng XF, et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson's disease, Parkinson's disease dementia, and dementia with Lewy bodies: systematic review with metaanalysis and trial sequential analysis. J Neurol Neurosurg Psychiatry. 2015; 86:135–143.

5). Stinton C, McKeith I, Taylor JP, Lafortune L, Mioshi E, Mak E, et al. Pharmacological management of lewy body dementia: a systematic review and metaanalysis. Am J Psychiatry. 2015; 172:731–742.

6). Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Alzheimer's Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005; 352:2379–2388.

7). Salloway S, Ferris S, Kluger A, Goldman R, Griesing T, Kumar D, et al. Donepezil 401 Study Group. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 2004; 63:651–657.

8). Doody RS, Ferris SH, Salloway S, Sun Y, Goldman R, Watkins WE, et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009; 72:1555–1561.

9). Kertesz A, Morlog D, Light M, Blair M, Davidson W, Jesso S, et al. Galantamine in frontotemporal dementia and primary progressive aphasia. Dement Geriatr Cogn Disord. 2008; 25:178–185.

10). Mendez MF, Shapira JS, McMurtray A, Licht E. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry. 2007; 15:84–87.

11). Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. 2006. CD005593.

12). Cummings JL. Use of cholinesterase inhibitors in clinical practice: evidence-based recommendations. Am J Geriatr Psychiatry. 2003; 11:131–145.

13). Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004; 292:2901–2908.
14). Wilcock G, Howe I, Coles H, Lilienfeld S, Truyen L, Zhu Y, et al. GAL-GBR-2 Study Group. A longterm comparison of galantamine and donepezil in the treatment of Alzheimer's disease. Drugs Aging. 2003; 20:777–789.

15). Ancoli-Israel S, Amatniek J, Ascher S, Sadik K, Ramaswamy K. Effects of galantamine versus donepezil on sleep in patients with mild to moderate Alzheimer disease and their caregivers: a double-blind, head-to-head, randomized pilot study. Alzheimer Dis Assoc Disord. 2005; 19:240–245.
16). Bullock R, Bergman H, Touchon J, Gambina G, He Y, Nagel J, et al. Effect of age on response to rivastigmine or donepezil in patients with Alzheimer's disease. Curr Med Res Opin. 2006; 22:483–494.

17). Campbell NL, Perkins AJ, Gao S, Skaar TC, Li L, Hendrie HC, et al. Adherence and tolerability of Alzheimer's Disease medications: a pragmatic randomized trial. J Am Geriatr Soc. 2017; 65:1497–1504.

18). Farlow MR, Salloway S, Tariot PN, Yardley J, Moline ML, Wang Q, et al. Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer's disease: a 24-week, randomized, double-blind study. Clin Ther. 2010; 32:1234–1251.

19). Gill SS, Anderson GM, Fischer HD, Bell CM, Li P, Normand SL, et al. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med. 2009; 169:867–873.
20). Winblad B, Cummings J, Andreasen N, Grossberg G, Onofrj M, Sadowsky C, et al. A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer's disease–rivastigmine patch versus capsule. Int J Geriatr Psychiatry. 2007; 22:456–467.
21). Sheffrin M, Miao Y, Boscardin WJ, Steinman MA. Weight loss associated with cholinesterase inhibitors in individuals with dementia in a national healthcare system. J Am Geriatr Soc. 2015; 63:1512–1518.

22). Soysal P, Isik AT, Stubbs B, Solmi M, Volpe M, Luchini C, et al. Acetylcholinesterase inhibitors are associated with weight loss in older people with dementia: a systematic review and metaanalysis. J Neurol Neurosurg Psychiatry. 2016; 87:1368–1374.

23). Kim DH, Brown RT, Ding EL, Kiel DP, Berry SD. Dementia medications and risk of falls, syncope, and related adverse events: metaanalysis of randomized controlled trials. J Am Geriatr Soc. 2011; 59:1019–1031.

24). Lee JY, Lee DW, Cho SJ, Na DL, Jeon HJ, Kim SK, et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol. 2008; 21:104–110.

25). Kang SJ, Choi SH, Lee BH, Jeong Y, Hahm DS, Han IW, et al. Care-giver-Administered Neuropsychiatric Inventory (CGA-NPI). J Geriatr Psychiatry Neurol. 2004; 17:32–35.

26). Rainer M, Mucke HA, Krüger-Rainer C, Kraxberger E, Haushofer M, Jellinger KA. Cognitive relapse after discontinuation of drug therapy in Alzheimer's disease: cholinesterase inhibitors versus nootropics. J Neural Transm (Vienna). 2001; 108:1327–1333.

27). O'Regan J, Lanctôt KL, Mazereeuw G, Herrmann N. Cholinesterase inhibitor discontinuation in patients with Alzheimer's disease: a metaanalysis of randomized controlled trials. J Clin Psychiatry. 2015; 76:e1424–e1431.
28). Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, et al. Donepezil and memantine for moderate-to-severe Alzheimer's disease. N Engl J Med. 2012; 366:893–903.

29). Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, et al. Nursing home placement in the Donepezil and Memantine in Moderate to Severe Alzheimer's Disease (DOMINO-AD) trial: secondary and post-hoc analyses. Lancet Neurol. 2015; 14:1171–1181.

30). Pariente A, Fourrier-Réglat A, Bazin F, Ducruet T, Dartigues JF, Drag-omir A, et al. Effect of treatment gaps in elderly patients with dementia treated with cholinesterase inhibitors. Neurology. 2012; 78:957–963.

31). Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ. Memantine Study Group. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003; 348:1333–1341.

32). Orrego F, Villanueva S. The chemical nature of the main central excitatory transmitter: a critical appraisal based upon release studies and synaptic vesicle localization. Neuroscience. 1993; 56:539–555.

33). Danysz W, Parsons CG. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev. 1998; 50:597–664.
34). Lancelot E, Beal MF. Glutamate toxicity in chronic neurodegenerative disease. Prog Brain Res. 1998; 116:331–347.
35). Kornhuber J, Weller M, Schoppmeyer K, Riederer P. Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. J Neural Transm Suppl. 1994; 43:91–104.
36). Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006; 63:49–54.

37). Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004; 291:317–324.
38). Cummings JL, Schneider E, Tariot PN, Graham SM. Memantine MEM-MD-02 Study Group. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology. 2006; 67:57–63.

39). Atri A, Shaughnessy LW, Locascio JJ, Growdon JH. Longterm course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008; 22:209–221.

40). Lopez OL, Becker JT, Wahed AS, Saxton J, Sweet RA, Wolk DA, et al. Longterm effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009; 80:600–607.

41). McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006. CD003154.

42). Schneider LS, Dagerman KS, Higgins JP, McShane R. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch Neurol. 2011; 68:991–998.

43). Ridha BH, Josephs KA, Rossor MN. Delusions and hallucinations in dementia with Lewy bodies: worsening with memantine. Neurology. 2005; 65:481–482.

44). Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008; 148:379–397.

45). Qaseem A, Snow V, Cross JT Jr, Forciea MA, Hopkins R Jr, Shekelle P, et al. American College of Physicians/American Academy of Family Physicians Panel on Dementia. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2008; 148:370–378.

46). Porsteinsson AP, Grossberg GT, Mintzer J, Olin JT. Memantine MEM-MD-12 Study Group. Memantine treatment in patients with mild to moderate Alzheimer's disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008; 5:83–89.

47). Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. The Alzheimer's Disease Cooperative Study. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. N Engl J Med. 1997; 336:1216–1222.

48). Dysken MW, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA. 2014; 311:33–44.
49). Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, et al. Alzheimer's Disease Cooperative Study. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol. 2012; 69:836–841.
50). Birks J, Flicker L. Selegiline for Alzheimer's disease. Cochrane Database Syst Rev. 2003. CD000442.

51). Henderson VW. Estrogen, cognition, and a woman's risk of Alzheimer's disease. Am J Med. 1997; 103:11S–18S.

52). Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003; 289:2651–2662.

53). Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sher-win BB, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004; 291:2959–2968.

54). Wang PN, Liao SQ, Liu RS, Liu CY, Chao HT, Lu SR, et al. Effects of estrogen on cognition, mood, and cerebral blood flow in AD: a controlled study. Neurology. 2000; 54:2061–2066.
55). Henderson VW, Paganini-Hill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, et al. Estrogen for Alzheimer's disease in women: randomized, double-blind, placebo-controlled trial. Neurology. 2000; 54:295.

56). Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer's Disease Cooperative Study. JAMA. 2000; 283:1007–1015.
57). Rigaud AS, André G, Vellas B, Touchon J, Pere JJ. French Study Group. No additional benefit of HRT on response to rivastigmine in menopausal women with AD. Neurology. 2003; 60:148–149.

58). Asthana S, Baker LD, Craft S, Stanczyk FZ, Veith RC, Raskind MA, et al. High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology. 2001; 57:605–612.

59). LeBlanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: systematic review and metaanalysis. JAMA. 2001; 285:1489–1499.

60). Rogers J, Webster S, Lue LF, Brachova L, Civin WH, Emmerling M, et al. Inflammation and Alzheimer's disease pathogenesis. Neurobiol Aging. 1996; 17:681–686.

61). Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, et al. In-vivo measurement of activated microglia in dementia. Lancet. 2001; 358:461–467.

62). Andersen K, Launer LJ, Ott A, Hoes AW, Breteler MM, Hofman A. Do nonsteroidal anti-inflammatory drugs decrease the risk for Alzheimer's disease? The Rotterdam Study. Neurology. 1995; 45:1441–1445.

63). Firuzi O, Praticò D. Coxibs and Alzheimer's disease: should they stay or should they go? Ann Neurol. 2006; 59:219–228.

64). Scharf S, Mander A, Ugoni A, Vajda F, Christophidis N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer's disease. Neurology. 1999; 53:197–201.

65). Van Gool WA, Weinstein HC, Scheltens P, Walstra GJ. Effect of hydroxychloroquine on progression of dementia in early Alzheimer's disease: an 18-month randomised, double-blind, placebo-controlled study. Lancet. 2001; 358:455–460.

66). Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, et al. Alzheimer's Disease Cooperative Study. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003; 289:2819–2826.
67). Reines SA, Block GA, Morris JC, Liu G, Nessly ML, Lines CR, et al. Rofecoxib Protocol 091 Study Group. Rofecoxib: no effect on Alzheimer's disease in a 1-year, randomized, blinded, controlled study. Neurology. 2004; 62:66–71.

68). AD2000 Collaborative Group. Bentham P, Gray R, Sellwood E, Hills R, Crome P, et al. Aspirin in Alzheimer's disease (AD2000): a randomised open-label trial. Lancet Neurol. 2008; 7:41–49.
69). Jaturapatporn D, Isaac MG, McCleery J, Tabet N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer's disease. Cochrane Database Syst Rev. 2012. CD006378.

70). Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2007. CD003120.

71). Sparks DL, Sabbagh MN, Connor DJ, Lopez J, Launer LJ, Browne P, et al. Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol. 2005; 62:753–757.
72). Sano M, Bell KL, Galasko D, Galvin JE, Thomas RG, van Dyck CH, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011; 77:556–563.

73). Feldman HH, Doody RS, Kivipelto M, Sparks DL, Waters DD, Jones RW, et al. LEADe Investigators. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010; 74:956–964.

74). Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, et al. Alzheimer Disease Cooperative Study. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008; 300:1774–1783.
75). Daiello LA, Gongvatana A, Dunsiger S, Cohen RA, Ott BR. Alzheimer's Disease Neuroimaging Initiative. Association of fish oil supplement use with preservation of brain volume and cognitive function. Alzheimers Dement. 2015; 11:226–235.

76). Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010; 304:1903–1911.
77). Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, Basun H, Faxén-Irving G, Garlind A, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: omegAD study: a randomized double-blind trial. Arch Neurol. 2006; 63:1402–1408.
78). Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer's disease: past, present and future. Neuropharmacology. 2014; 76 Pt A:27–50.

79). Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012; 69:29–38.
80). Harrington C, Sawchak S, Chiang C, Davies J, Donovan C, Saunders AM, et al. Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer's disease: two phase 3 studies. Curr Alzheimer Res. 2011; 8:592–606.

81). Cummings J, Lee G, Mortsdorf T, Ritter A, Zhong K. Alzheimer's disease drug development pipeline: 2017. Alzheimers Dement (N Y). 2017; 3:367–384.

82). Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012; 488:96–99.

83). Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer's disease from normal and pathologic aging. Exp Neurol. 1999; 158:328–337.
84). Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. AN1792(QS-21)-201 Study Team. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005; 64:1553–1562.
85). Patton RL, Kalback WM, Esh CL, Kokjohn TA, Van Vickle GD, Lu-ehrs DC, et al. Amyloid-beta peptide remnants in AN-1792-immunized Alzheimer's disease patients: a biochemical analysis. Am J Pathol. 2006; 169:1048–1063.
86). Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, et al. AN1792(QS-21)-201 Study. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005; 64:1563–1572.
Go to : 
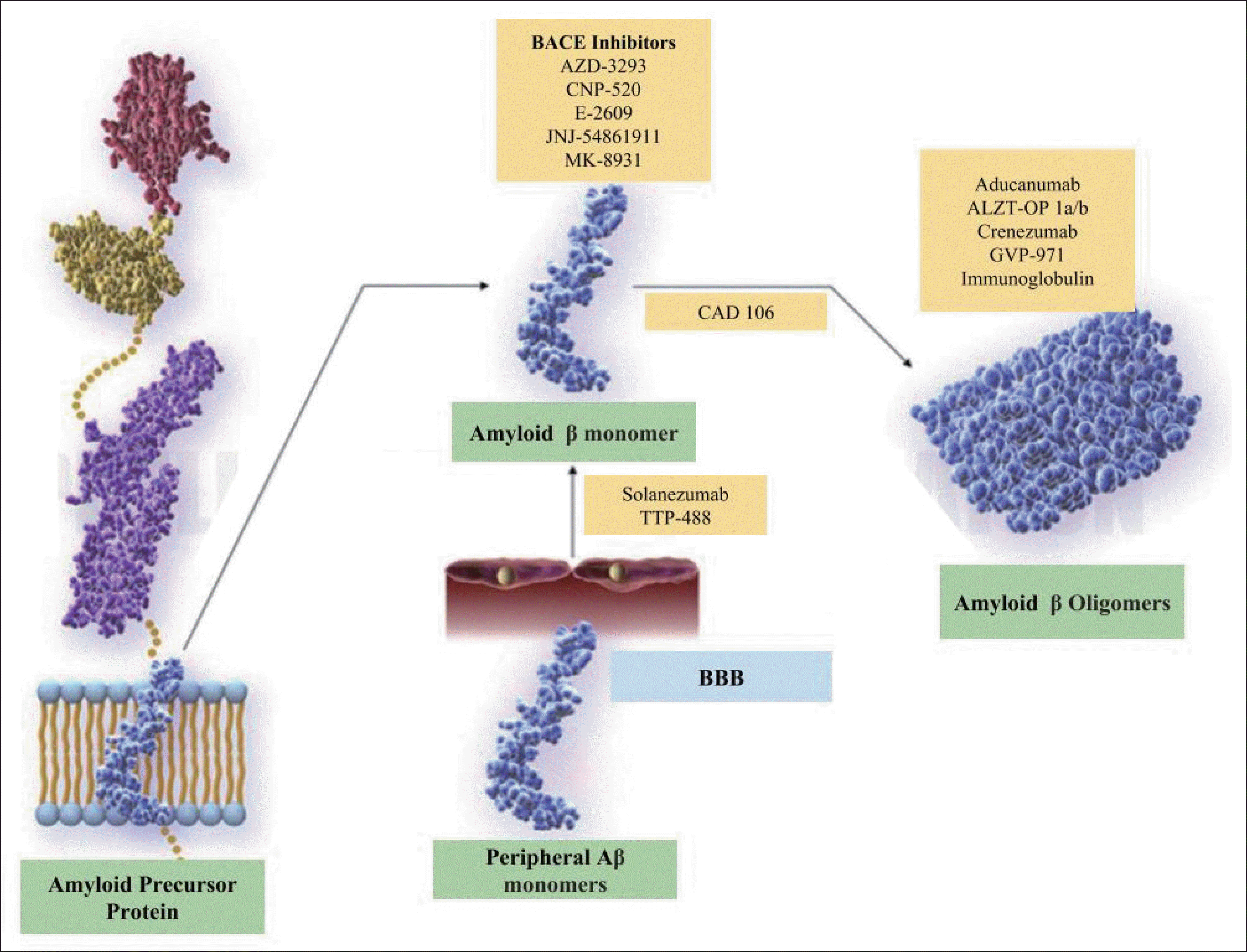 | Fig. 1.Production of amyloid beta and the formation of amyloid beta oligomers. Modified from Cummings et al. Alzheimers Dement 2017;3:367-384.81)
|
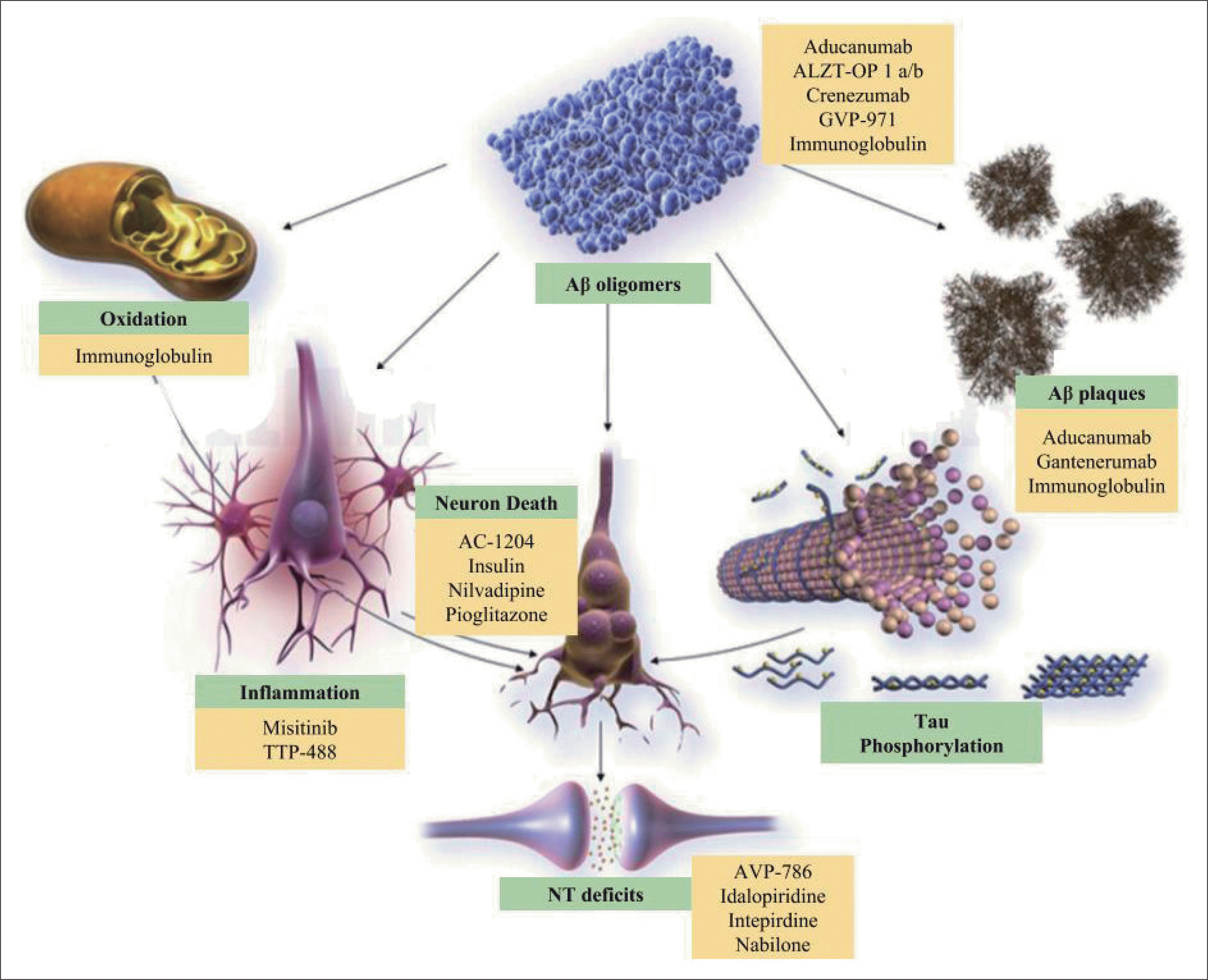 | Fig. 2.Influence of amyloid beta oligomers with the mitochondrial oxidation, neuroinflammation, neuronal death and neurotransmitter de-81)
|
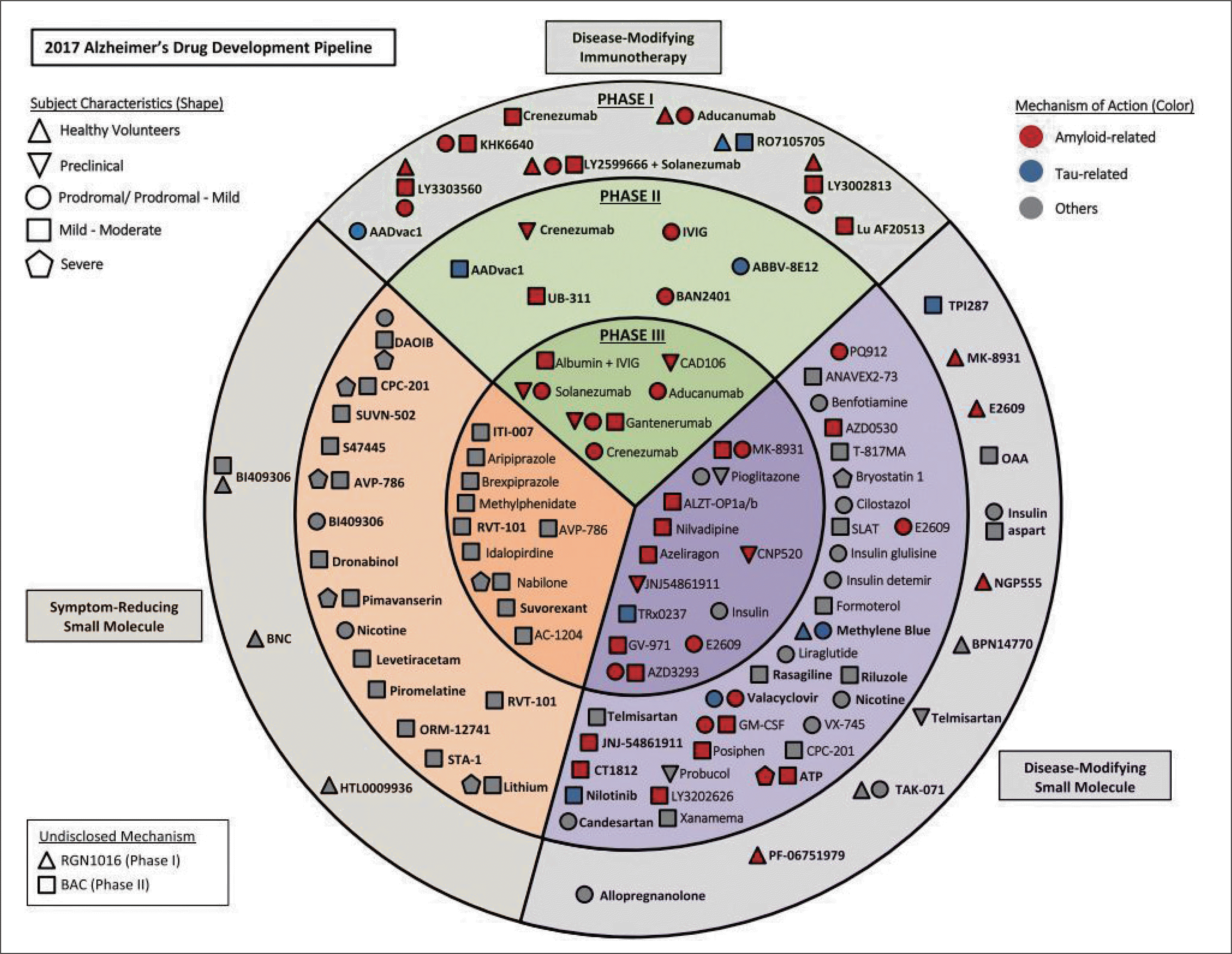 | Fig. 3.Agents in clinical trials for the treatment of Alzheimer's disease. Modified from Cummings et al. Alzheimers Dement 2017;3:367-384. 81) ATP : Adenosine triphosphate, BNC : Bisnorcymserine, GM-CSF : Granulocyte-macrophage colony-stimulating factor, OAA : Ox-aloacetate, IVIG : Intravenous immunoglobulin, SLAT : Simvastatin 1 L-arginine 1 tetrahydrobiopterin. |
Table 1.
Therapeutic strategies in Alzheimer's disease
Table 2.
Clinical trials of amyloid or tau based immuno-therapeutics




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download