Abstract
Alzheimer's disease (AD) is a debilitating syndrome with cognitive decline and impairment in daily activities. Although clinical assessment forms the basis for diagnosing AD, structural and functional brain imaging tools have been known to enhance accuracy of differential diagnosis and prognosis prediction by presenting structural and functional signatures for AD. Associated with the important role of brain imaging in diagnosing and treating AD, brain imaging has been recommended in the current diagnostic guidelines of AD. Visual rating scales, a cost-effective diagnostic tool, have been known to assess atrophy and functional changes in patients with cognitive impairment as accurate as quantitative assessment. In this regard, visual rating scales for brain imaging interpretation could be useful in clinical settings. In this review, we interpret structural and functional brain imaging results with standardized visual rating scales, and review recent findings concerning brain imaging tools for differential diagnosing and predicting prognosis of AD.
REFERENCES
1). Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Alzheimer Dis Assoc Disord. 1988; 2:134.
2). Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985; 82:4245–4249.

3). Westman E, Simmons A, Muehlboeck JS, Mecocci P, Vellas B, Tsola-ki M, et al. AddNeuroMed consortium; Alzheimer's Disease Neuroimaging Initiative. AddNeuroMed and ADNI: similar patterns of Alzheimer's atrophy and automated MRI classification accuracy in Europe and North America. Neuroimage. 2011; 58:818–828.

4). Henneman WJ, Sluimer JD, Barnes J, van der Flier WM, Sluimer IC, Fox NC, et al. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009; 72:999–1007.

5). Kantarci K, Jack CR. Neuroimaging in Alzheimer disease. Medina LS, Blackmore CC, editors. editors.Evidence-based imaging: optimizing imaging in patient care. 1st ed.New York, NY: Springer;2006. p. 142–159.

6). Harper L, Barkhof F, Fox NC, Schott JM. Using visual rating to diagnose dementia: a critical evaluation of MRI atrophy scales. J Neurol Neurosurg Psychiatry. 2015; 86:1225–1233.

7). de Leon MJ, George AE, Stylopoulos LA, Smith G, Miller DC. Early marker for Alzheimer's disease: the atrophic hippocampus. Lancet. 1989; 2:672–673.

8). Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992; 55:967–972.

9). Galton CJ, Gomez-Anson B, Antoun N, Scheltens P, Patterson K, Graves M, et al. Temporal lobe rating scale: application to Alzheimer's disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2001; 70:165–173.

10). Kaneko T, Kaneko K, Matsushita M, Kadoya M, Ihara N, Ryokawa A, et al. New visual rating system for medial temporal lobe atrophy: a simple diagnostic tool for routine examinations. Psychogeriatrics. 2012; 12:88–92.

11). Cavallin L, L⊘ken K, Engedal K, Oksengård AR, Wahlund LO, Bronge L, et al. Overtime reliability of medial temporal lobe atrophy rating in a clinical setting. Acta Radiol. 2012; 53:318–323.

12). Cavallin L, Bronge L, Zhang Y, Oksengård AR, Wahlund LO, Frati-glioni L, et al. Comparison between visual assessment of MTA and hippocampal volumes in an elderly, non-demented population. Acta Radiol. 2012; 53:573–579.

13). McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984; 34:939–944.

14). Barber R, Gholkar A, Scheltens P, Ballard C, McKeith IG, O'Brien JT. Medial temporal lobe atrophy on MRI in dementia with Lewy bodies. Neurology. 1999; 52:1153–1158.

15). Pasquier F, Leys D, Weerts JG, Mounier-Vehier F, Barkhof F, Scheltens P. Inter- and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur Neurol. 1996; 36:268–272.
16). Henneman WJ, Sluimer JD, Cordonnier C, Baak MM, Scheltens P, Barkhof F, et al. MRI biomarkers of vascular damage and atrophy predicting mortality in a memory clinic population. Stroke. 2009; 40:492–498.

17). Ferreira D, Cavallin L, Granberg T, Lindberg O, Aguilar C, Mecocci P, et al. AddNeuroMed consortium and for the Alzheimer's Disease Neuroimaging Initiative. Quantitative validation of a visual rating scale for frontal atrophy: associations with clinical status, APOE e4, CSF biomarkers and cognition. Eur Radiol. 2016; 26:2597–2610.

18). McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7:263–269.

19). Barkhof F, Fox NC, Bastos-Leite AJ, Scheltens P. Neuroimaging in dementia. Berlin: Springer Science & Business Media;2011.
20). Scheltens P, Pasquier F, Weerts JG, Barkhof F, Leys D. Qualitative assessment of cerebral atrophy on MRI: inter- and intra-observer reproducibility in dementia and normal aging. Eur Neurol. 1997; 37:95–99.

21). Koedam EL, Lehmann M, van der Flier WM, Scheltens P, Pijnenburg YA, Fox N, et al. Visual assessment of posterior atrophy development of a MRI rating scale. Eur Radiol. 2011; 21:2618–2625.

22). Lehmann M, Koedam EL, Barnes J, Bartlett JW, Ryan NS, Pijnenburg YA, et al. Posterior cerebral atrophy in the absence of medial temporal lobe atrophy in pathologically-confirmed Alzheimer's disease. Neurobiol Aging [serial online] 2012 Mar [cited 2018 Feb 1];33(3): 627.e1-627.e12. Available from:. http://www.neurobiologyofaging.org/article/S0197-4580(11)00118-7/fulltext.
23). Inzitari D, Simoni M, Pracucci G, Poggesi A, Basile AM, Chabriat H, et al. LADIS Study Group. Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes: the LADIS Study. Arch Intern Med. 2007; 167:81–88.
24). Inzitari D, Pracucci G, Poggesi A, Carlucci G, Barkhof F, Chabriat H, et al. LADIS Study Group. Changes in white matter as determinant of global functional decline in older independent outpatients: three year followup of LADIS (leukoaraiosis and disability) study cohort. BMJ. 2009; 339:b2477.

25). Sokoloff L. The deoxyglucose method for the measurement of local glucose utilization and the mapping of local functional activity in the central nervous system. Int Rev Neurobiol. 1981; 22:287–333.

26). Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013; 12:207–216.

28). Ossenkoppele R, Tolboom N, Foster-Dingley JC, Adriaanse SF, Boellaard R, Yaqub M, et al. Longitudinal imaging of Alzheimer pathology using [11C]PIB, [18F]FDDNP and [18F]FDG PET. Eur J Nucl Med Mol Imaging. 2012; 39:990–1000.

29). Protas HD, Chen K, Langbaum JB, Fleisher AS, Alexander GE, Lee W, et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol. 2013; 70:320–325.

30). Förster S, Grimmer T, Miederer I, Henriksen G, Yousefi BH, Graner P, et al. Regional expansion of hypometabolism in Alzheimer's disease follows amyloid deposition with temporal delay. Biol Psychiatry. 2012; 71:792–797.

31). Mosconi L, Tsui WH, De Santi S, Li J, Rusinek H, Convit A, et al. Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology. 2005; 64:1860–1867.

32). Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer's disease, and other dementias. J Nucl Med. 2008; 49:390–398.

33). Hoffman JM, Welsh-Bohmer KA, Hanson M, Crain B, Hulette C, Earl N, et al. FDG PET imaging in patients with pathologically verified dementia. J Nucl Med. 2000; 41:1920–1928.
34). Arnáiz E, Jelic V, Almkvist O, Wahlund LO, Winblad B, Valind S, et al. Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. Neuroreport. 2001; 12:851–855.

35). Chételat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer's disease? Neurology. 2003; 60:1374–1377.

36). Anchisi D, Borroni B, Franceschi M, Kerrouche N, Kalbe E, Beuthien-Beumann B, et al. Heterogeneity of brain glucose metabolism in mild cognitive impairment and clinical progression to Alzheimer disease. Arch Neurol. 2005; 62:1728–1733.

37). Drzezga A, Grimmer T, Riemenschneider M, Lautenschlager N, Siebner H, Alexopoulus P, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J Nucl Med. 2005; 46:1625–1632.
38). Minoshima S, Foster NL, Sima AA, Frey KA, Albin RL, Kuhl DE. Alzheimer's disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol. 2001; 50:358–365.

39). Foster NL, Heidebrink JL, Clark CM, Jagust WJ, Arnold SE, Barbas NR, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer's disease. Brain. 2007; 130(Pt 10):2616–2635.

40). Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006; 66:1837–1844.

41). Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salva-do O, et al. Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013; 12:357–367.

42). Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, et al. Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing; Alzheimer's Disease Neuroimaging Initiative; Alzheimer's Disease Cooperative Study. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014; 71:961–970.
43). Lim YY, Pietrzak RH, Ellis KA, Jaeger J, Harrington K, Ashwood T, et al. Rapid decline in episodic memory in healthy older adults with high amyloid-β. J Alzheimers Dis. 2013; 33:675–679.

44). Klunk WE, Wang Y, Huang GF, Debnath ML, Holt DP, Mathis CA. Uncharged thioflavin-T derivatives bind to amyloid-beta protein with high affinity and readily enter the brain. Life Sci. 2001; 69:1471–1484.

45). Heurling K, Leuzy A, Zimmer ER, Lubberink M, Nordberg A. Imaging β-amyloid using [(18)F]flutemetamol positron emission tomography: from dosimetry to clinical diagnosis. Eur J Nucl Med Mol Imaging. 2016; 43:362–373.

46). Bullich S, Seibyl J, Catafau AM, Jovalekic A, Koglin N, Barthel H, et al. Optimized classification of 18F-Florbetaben PET scans as positive and negative using an SUVR quantitative approach and comparison to visual assessment. Neuroimage Clin. 2017; 15:325–332.

47). Landau SM, Breault C, Joshi AD, Pontecorvo M, Mathis CA, Jagust WJ, et al. Alzheimer's Disease Neuroimaging Initiative. Amyloid-β imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med. 2013; 54:70–77.

48). Villemagne VL, Mulligan RS, Pejoska S, Ong K, Jones G, O'Keefe G, et al. Comparison of 11C-PiB and 18F-florbetaben for Aβ imaging in ageing and Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2012; 39:983–989.

49). Curtis C, Gamez JE, Singh U, Sadowsky CH, Villena T, Sabbagh MN, et al. Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density. JAMA Neurol. 2015; 72:287–294.

50). Jennings D, Seibyl J, Sabbagh M, Lai F, Hopkins W, Bullich S, et al. Age dependence of brain β-amyloid deposition in Down syndrome: an [18F]florbetaben PET study. Neurology. 2015; 84:500–507.

51). Sabri O, Sabbagh MN, Seibyl J, Barthel H, Akatsu H, Ouchi Y, et al. Florbetaben Phase 3 Study Group. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer's disease: Phase 3 Study. Alzheimers Dement. 2015; 11:964–974.

52). Rabinovici GD, Rosen HJ, Alkalay A, Kornak J, Furst AJ, Agarwal N, et al. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology. 2011; 77:2034–2042.

53). Siderowf A, Pontecorvo MJ, Shill HA, Mintun MA, Arora A, Joshi AD, et al. PET imaging of amyloid with Florbetapir F 18 and PET imaging of dopamine degeneration with 18F-AV-133 (florbenazine) in patients with Alzheimer's disease and Lewy body disorders. BMC Neurol. 2014; 14:79.

54). Jack CR Jr, Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, et al. Alzheimer's Disease Neuroimaging Initiative. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain. 2010; 133:3336–3348.

Fig. 1.
Scheltens scale for medial temporal atrophy. A : Coronal images of the hippocampus. B : Coronal brain images of Scheltens scale for medial temporal lobe atrophy. Image courtesy of Frederick Barkhof and Alzheimer Centre and Image Analysis Centre, Vrije Universiteit Medical Center, Amsterdam, Netherlands. Score 0 : No atrophy, Score 1 : Only widening of choroid fissure, Score 2 : Also widening of temporal horn of lateral ventricle, Score 3 : Moderate loss of hippocampal volume (decrease in height), Score 4 : Severe volume loss of hippocampus.
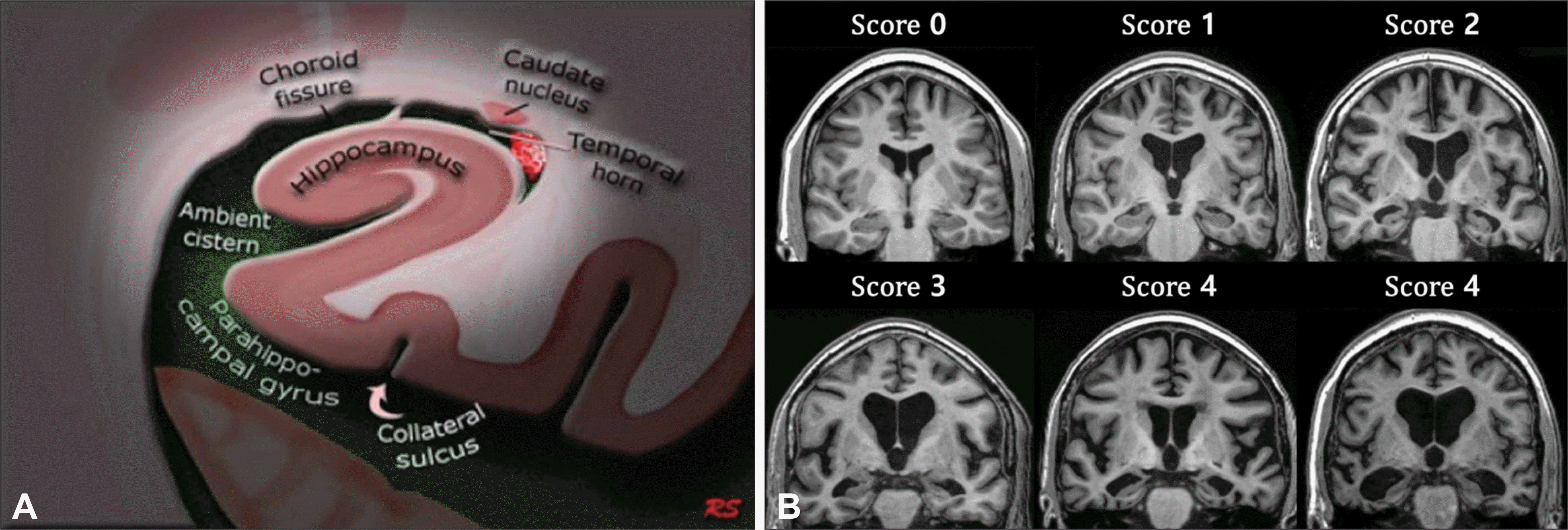
Fig. 2.
Transaxial brain images of Pasquier scale for global cortical atrophy. Image courtesy of Jonathan M Schott and Dementia Research Centre, University College London Institute of Neurology. London, UK. Adapted from Harper L et al. J Neurol Neurosurg Psychiatry 2015;86:1125-1233.6) Score 0 : No cortical atrophy, Score 1 : Mild atrophy : opening of sulci, Score 2 : Moderate atrophy : volume loss of gyri, Score 3 : Severe (end-stage) atrophy : ‘knife blade’ atrophy.
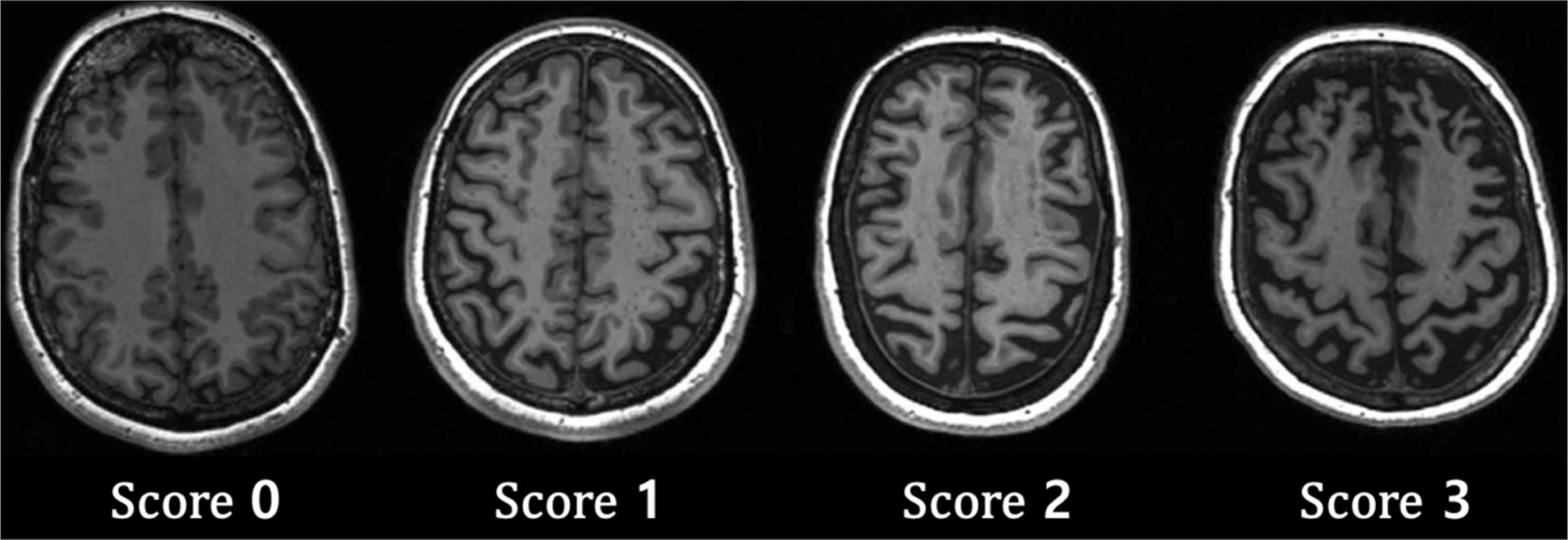
Fig. 3.
Brain images of Koedam scale for posterior cortical atrophy. Koedam scale is rated by evaluating atrophy of posterior cingulate sulcus, precuneus, parieto-occipital sulcus, and cortex of parietal lobe. ∗ : Extreme widening of the posterior cingulate, † : Widening of parieto-occipital sulci. Image courtesy of Frederick Barkhof and Alzheimer Centre and Image Analysis Centre, Vrije Universiteit Medical Center, Amsterdam, Netherlands.
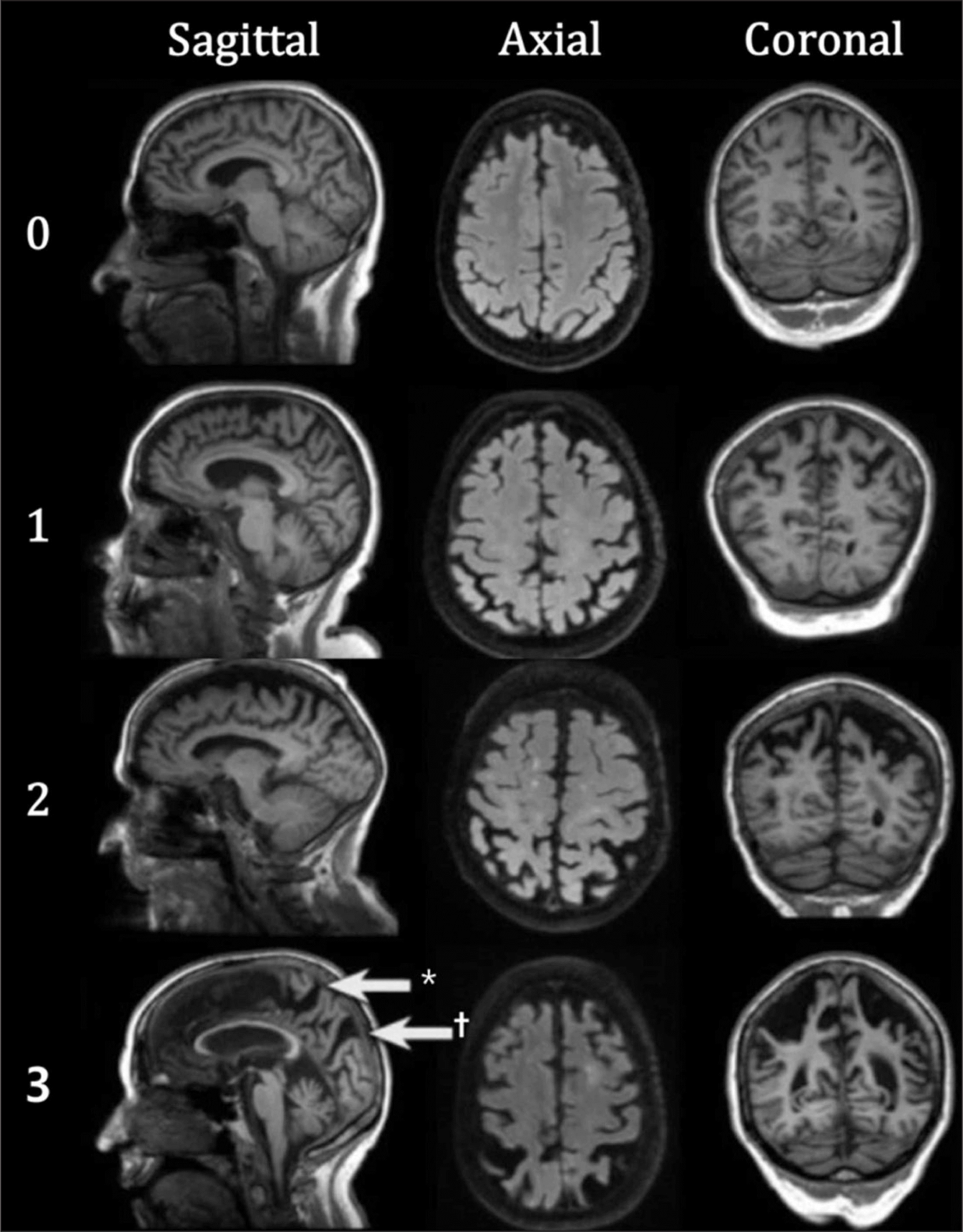
Fig. 4.
Transaxial brain images of Fazeka scale for rating of white matter hyperintensities. Image courtesy of Hyun Kook Lim, Department of Psychiatry, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. Fazeka 0 : No white matter hyperintensity, Fazeka 1 : Punctate lesions in the deep white matter with a maximum diameter of 9 mm for a single lesion and of 20 mm for grouped lesions, Fazeka 2 : Early confluent lesions of 10–20 mm single lesions and > 20 mm grouped lesions, in any diameter, and no more than connecting bridges between the individual lesions, Fazeka 3 : Single lesions or confluent areas of hyperintensity of ≥20 mm in any diameter.
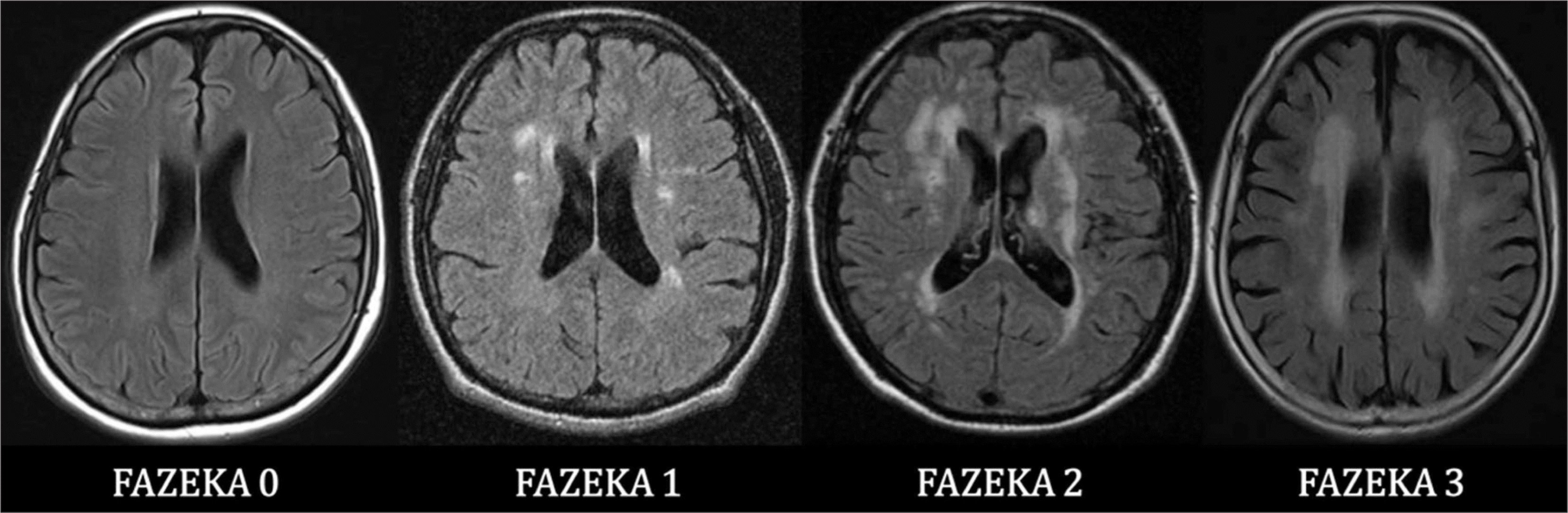
Fig. 5.
Normal 18F-fludeoxyglucose positron emission tomography scans. Yellow labeled regions display some asymmetry due to normal variations. A : 3 orthogonal scans with crosshairs displaying mutual references of cut location. B : Transaxial and coronal scans representing grey matter structures which guide anatomical orientation. Image courtesy of Herholz, K, Wolfson Molecular Imaging Centre and Manchester Academic Health Science Centre, University of Manchester, Manchester, UK. IF : Inter hemispheric fissure, CC : Corpus callosum, CG : Cingulate gyrus, OP : Occipital pole, LPFC : Lateral prefrontal cortex, PCN : Precuneus, PCC : Posterior cingulate cortex, TH : Thalamus, PT : Putamen, CD : Caudate nucleus, HG : Heschl's gyrus, TL : Temporal lobe, CBL : Cerebellum, EM : Eye muscle, HP : Hippocampus.
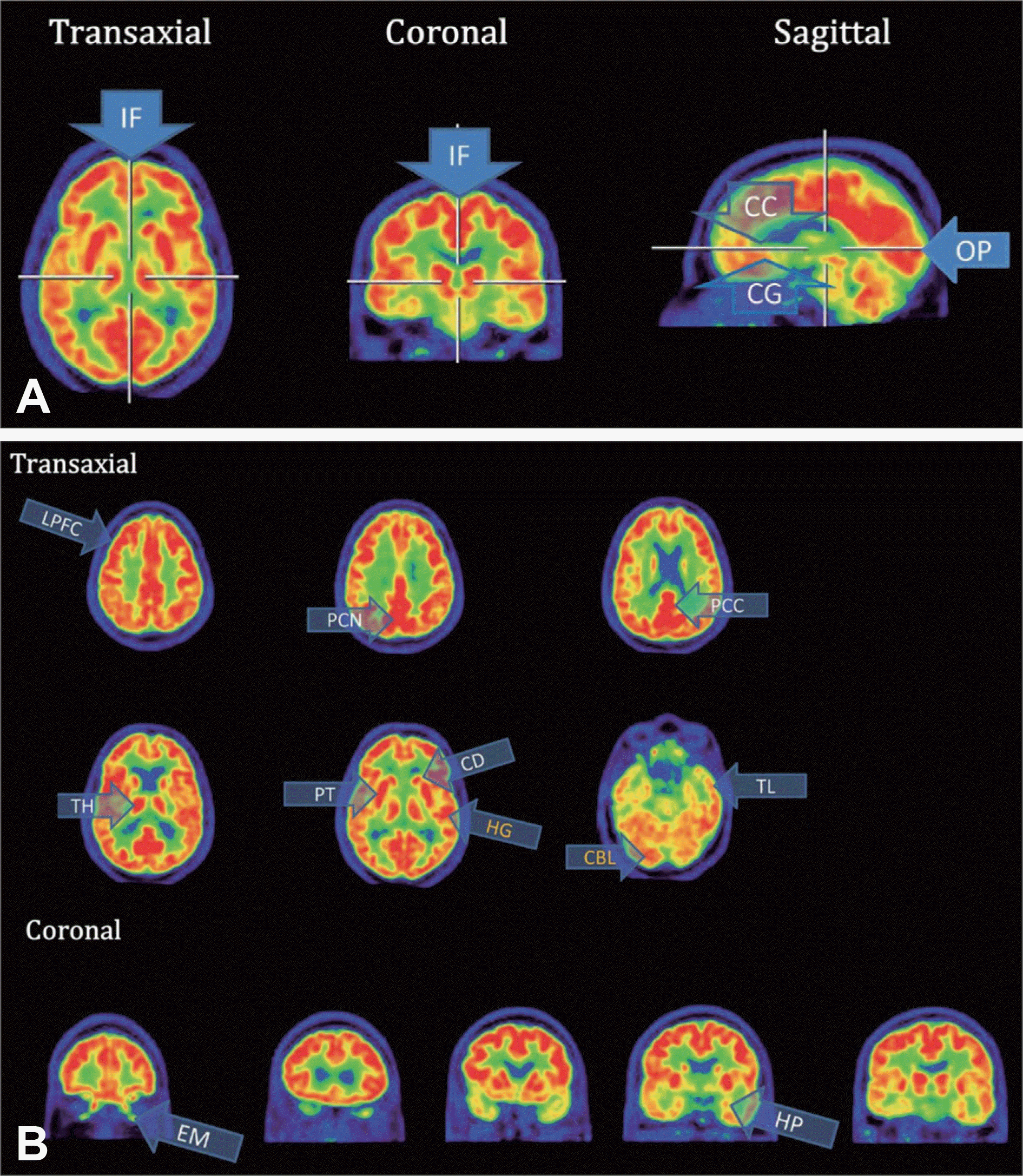
Fig. 6.
18F-fludeoxyglucose positron emission tomography scans in the trajectory of Alzheimer's disease. A : Orthogonal scans showing normal high activity of posterior cingulate cortex. B : Orthogonal scans displaying reduced metabolism in right posterior cingulate cortex in mild cognitive impairment patient. C : Transaxial scans representing reduced metabolic activity in parietal and temporal lobes in Alzheimer's disease patient. Image courtesy of Herholz, K, Wolfson Molecular Imaging Centre and Manchester Academic Health Science Centre, University of Manchester, Manchester, UK. CG : Cingulate gyrus, CC : Corpus callosum, ACC : Anterior cingulate cortex, PCC : Posterior cingulate cortex, POS : Parieto-occipital sulci, PCN : Precuneus, LPFC : Lateral prefrontal cortex.
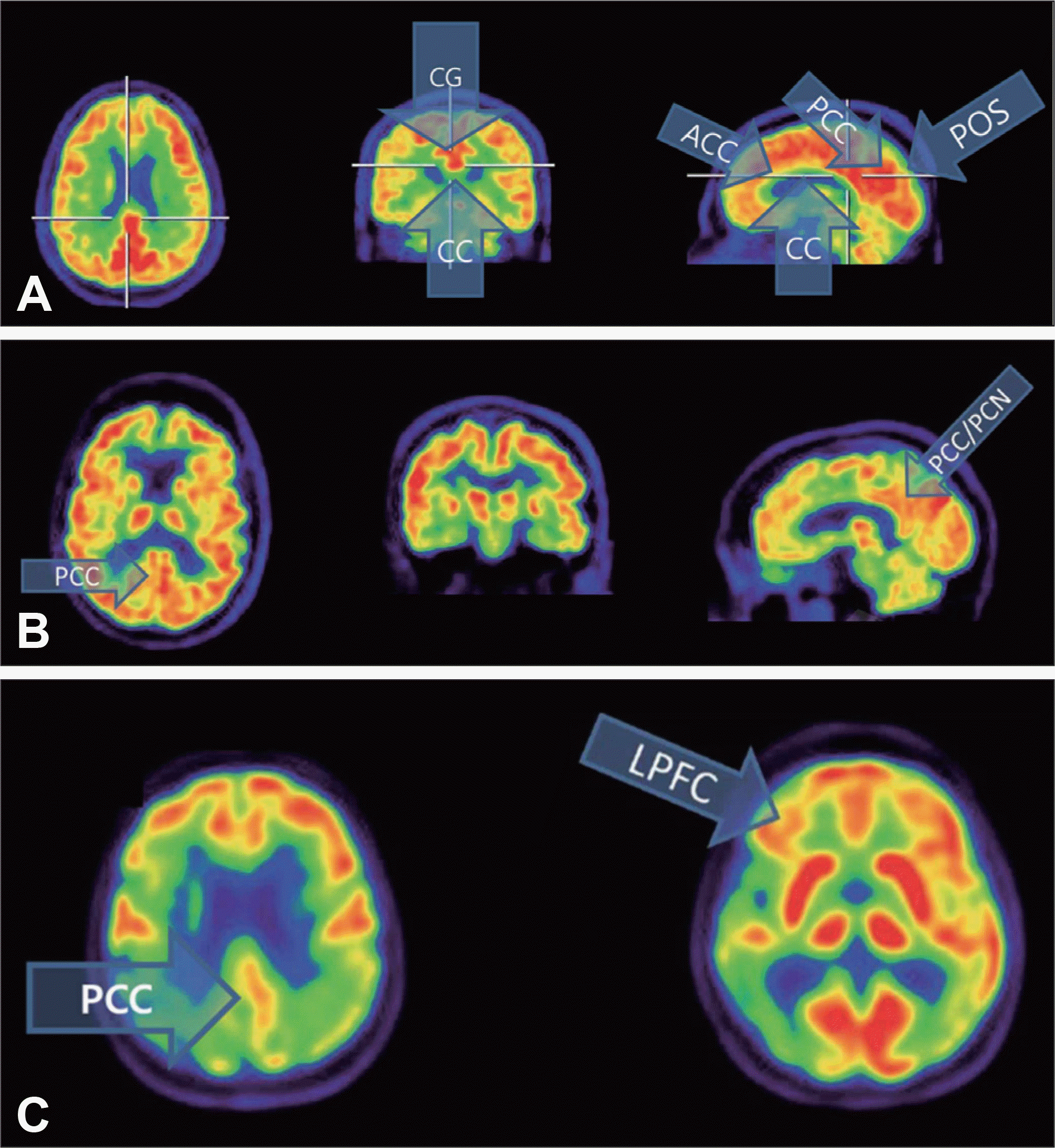
Fig. 7.
Normal and positive 18F-flutemetamol scans in regions of interest. A : Frontal lobe. B : Posterior cingulate cortex and precuneus. C : Lateral temporal lobe. D : Parietal lobe. E : Striatum. Image courtesy of Hyun Kook Lim, Department of Psychiatry, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
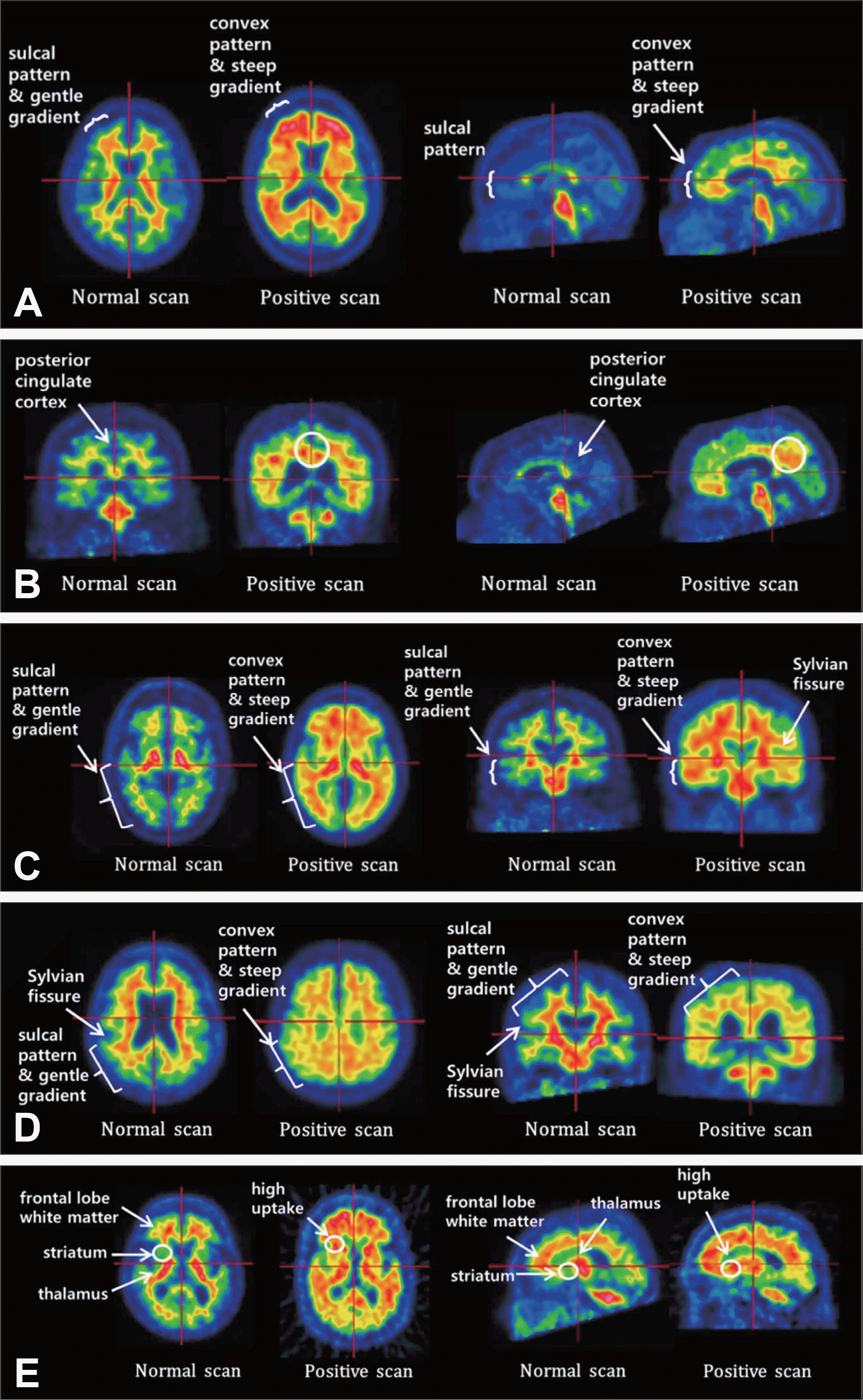
Fig. 8.
Normal and positive 18F-florbetaben scans in regions of interest. Cerebellum : Contrast between white matter (arrows) and grey matter is displayed in both normal and positive scans. Extracerebral tracer uptake in scalp and in posterior sagittal sinus (arrowheads) can be seen. Lateral temporal lobes : Positive scan shows smooth appearance of outer border (dashed line) in grey matter. Spiculated appearance of white matter (arrows) represents normal scan. Frontal lobes : Positive scan shows that tracer uptake has plumped appearance due to grey matter signal (dashed line). Spiculated appearance of white matter in frontal lobes (arrows) is seen in negative scan. Posterior cingulate/precuneus : Adjacent to splenium (arrows), region appears as hypointense hole (circle) in normal scan, whereas this hole is absent (circle) in positive scan. Parietal lobes : In positive scan, midline between parietal lobes is thinner. Cortical areas are filled up and show smoother appearance as uptake extends to outer rim. In normal scan, midline between parietal lobes can be easily detected (long arrows); white matter has spiculated appearance (short arrows) with less uptake to outer rim (dashed line). Image courtesy of the Piramal Physicians Training Site for Reading PET Amyloid Imaging. This research was originally published in JNM. John Seibyl et al. Impact of training method on the robustness of the visual assessment of 18F-florbetaben PET scans: results from a Phase-3 Study. J Nucl Med 2016;57:900-906. © by the Society of Nuclear Medicine and Molecular Imaging, Inc.
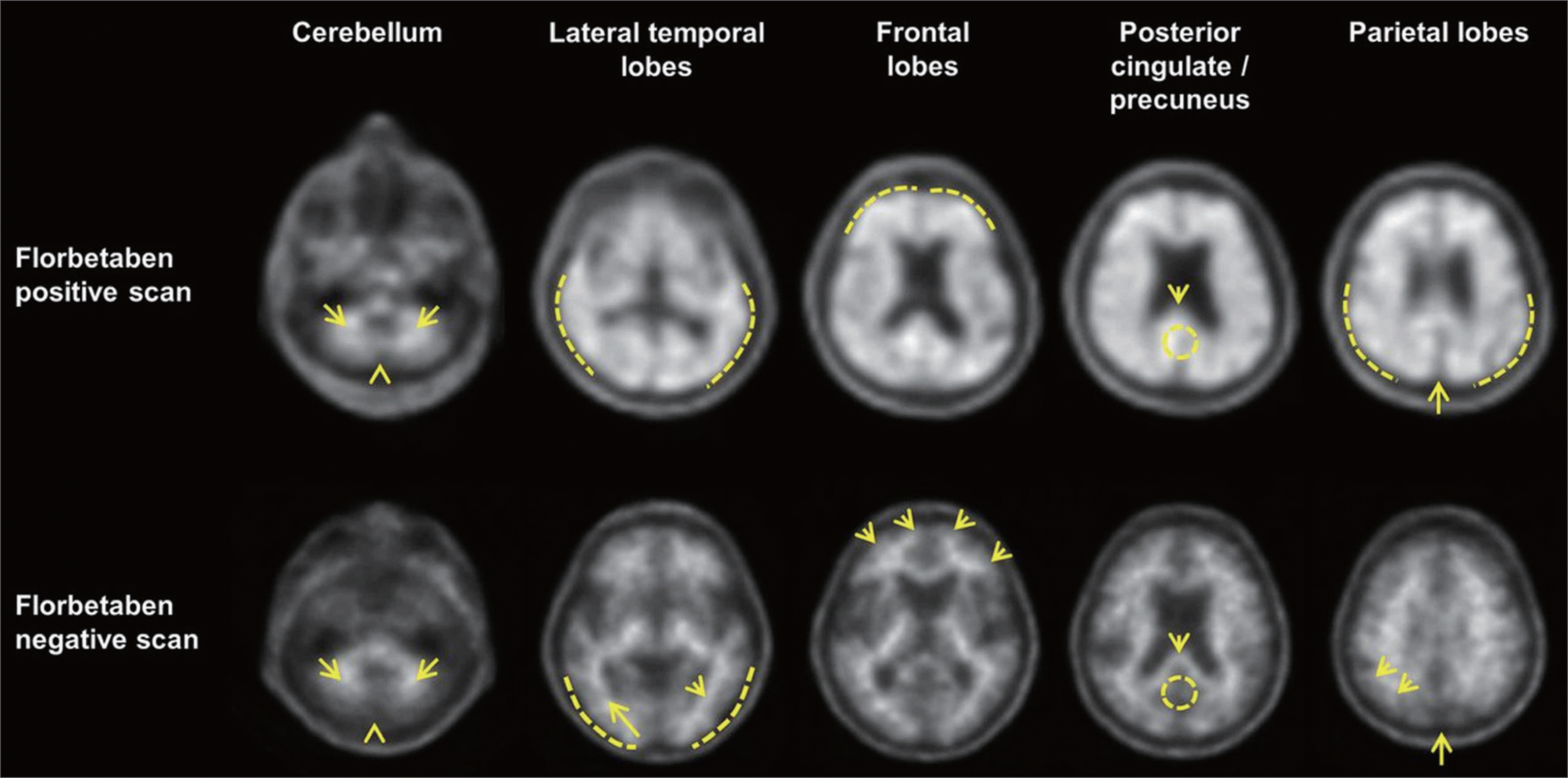
Table 1.
Scheltens scale for medial temporal atrophy
| Score | Width of choroid fissure | Width of temporal horn | Height of hippocampal formation |
|---|---|---|---|
| 0 | Normal | Normal | Normal |
| 1 | ↑ | Normal | Normal |
| 2 | ↑↑ | ↑ | ↓ |
| 3 | ↑↑↑ | ↑↑ | ↓↓ |
| 4 | ↑↑↑ | ↑↑↑ | ↓↓↓ |
Table 2.
Visual assessment method for 18F-florbetaben scans




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download