Abstract
Background and Objectives
Stent coarctoplasty has been approved as the treatment of choice for adult patients with coarctation of the aorta. We have evaluated the early and midterm clinical and procedural results after interventional coarctoplasty. Also, variables that can affect these results were evaluated.
Subjects and Methods
Gathering clinical, angiographic and procedural data, we evaluated the pre-specified outcomes, including procedural success, complications, the incidence of hypertension after coarctoplasty etc., after the procedure. The effect of pre-specified variables including aortic arch shape, coarctation type and etc. on the procedural result was evaluated.
Results
Between February 2005 through March 2014, 133 stent coarctoplasty procedures were performed. Median age was 23.5 years old (interquartile range [IQR]:19-28), and 105 (71.9%) were male. Nearly all of the patients were undergone stent coarctoplasty, mostly with cheatham platinum (CP) stents. There was no association between aortic arch morphology and acute procedural complications. Balloon length more than 40 mm (p=0.028), aorta diameter at the site of Coarctation larger than 2.35 mm (p=0.008) was associated with higher rate of restenosis during follow-up. Comparison between the prevalence of hypertension (HTN) before and after coarctoplasty showed a significant reduction in the prevalence of HTN (117 [91.4%] vs. 95 [74.2%] p<0.001).
Coarctation of the aorta is a relatively uncommon pathology with a prevalence of 5-8% among all congenital heart diseases.1)2) Fatal cardiovascular disorders are the consequences of this abnormality if the conditions are left untreated. Nowadays stent coarctoplasty is the best treatment option. It is minimally invasive with a success rate of about 90%.3) This treatment is effective in either initial or recurrent coarctation,4)5) with a relatively low incidence of complications. Acute complications of stent coarctoplasty include acute aortic complications (rupture, dissection, injuries to the branches of the aortic arch, etc.), access site complications (bleeding, hematoma, injuries of the common femoral and the common iliac arteries, etc.), embolic events and cerebrovascular accidents (CVA). Chronic complications include aneurysm formation at the stenting site and restenosis/recoarctation. The impact of this treatment on long-term cardiovascular consequences of coarctation (i.e. hypertension, [HTN], CVA, intracranial hemorrhage, heart failure) remains uncertain. We have conducted an observational study to evaluate the early and mid-term procedural and clinical outcomes of stent coarctoplasty. Also, anatomical and procedural variables that can affect the outcomes were evaluated.
This was an observational (cross-sectional) study, approved by the institutional review board at our hospital. Patient consent and subjects signed written consent whenever they have visited for the follow-up or prior to participation (as the study started before their participation).
Patients with diagnosed native coarctation of the aorta which had been undergone stent coarctoplasty between February 2005 through March 2014 in our hospital were entered into the study. The patient's clinical data were extracted from their documented files. Angiographic and procedural data were also extracted from saved angiography films.
Systolic blood pressure before, after and at the end of a follow-up visit along with a number of antihypertensive drugs used for them, the occurrence of CVA and any embolic events, heart failure, intracerebral hemorrhage (ICH) and mortality during follow-up.
There have been studies that found no relationships existed between the arch shape and the procedural result of coarctoplasty. However, there is no study to date that has evaluated the relationship between the distance from the origin of the subclavian artery (SCA) to the coarctation site and the procedural result. Technically it seems that the juxtaductal coarctation of the aorta, which has a very short or no distance to the SCA, are more difficult for stenting, because of the mismatch between proximal and distal landing zones in this anatomical category. Therefore, we gathered angiographic data, including the aortic arch shape, and the distance of SCA to coarctation site and categorized it into juxta and postductal coarctation.
Sheath size, pre-dilating balloon size (if used), stent type and size, balloon size and type used for stent deployment, balloon diameter: coarctation site diameter ratio, balloon diameter: aortic diameter ratio, systolic pressure gradient before and after coarctoplasty, procedural aortic complications, access site injuries, balloon rupture, stent-related complications especially stent migration and coverage of subclavian artery and successfulness of the procedure are gathered. The composite of acute procedural events includes acute embolic events, CVA, acute procedural aortic dissection, access site complications, mortality and balloon rupture.
A stenosis of thoracic aorta (usually as a congenital abnormality) with a trans-coarctation gradient of more than 20 mmHg. Of course, the gradient may be absent in cases with well-developed collaterals or interrupted aorta, so other imaging modalities apart from angiography may be mandated to confirm the diagnosis in such situation.
The distance from the subclavian artery origin to the proximal part of coarctation site is used. More than 5 mm distance defines the postductal type, and less than 5 mm distance is considered as post-ductal coarctation. This definition is based on the description has been made before (Fig. 1).
If the arch shape formed the two sides of a triangle, Gothic arch; if formed three sides of a square, Crenel arch and, if there was normal shaped arch circular arch have been considered. This categorization is on the basis of the width and height of the arch. Arch width is the line crossing the middle of ascending aorta continues toward descending aorta and arch height is defined as the distance between width line and the most upper part of the arch (Fig. 1).
Systolic blood pressure more than 140 mmHg and Diastolic blood pressure more than 90 mmHg was considered as HTN. Blood pressure was measured by using standard cuff and method for both upper limbs and left lower limb of each patient.
After right femoral artery cannulation, an Ilio-femoral angiogram is performed to evaluate their size. This is essential for prevention of injury to Ilio-femoral vessels as the large diameter sheaths should be introduced. Then a 0.035 inches' wire (usually a hydrophilic one) is passed through the coarctation (using a multipurpose catheter for support). In one case with interrupted aorta we've used a Brockenbrough needle to cross the lesion. In other cases, with interrupted aorta an upper limb access (usually right radial artery) is cannulated to define the anatomy of the lesion. We are using hand-crimped and pre-mounted cheatham platinum (CP) stents over BIB balloons (NuMED Inc., Hopkinton, NY, USA) for nearly all of the patients. Requiring sheath size for this approach is more than 12 French sheaths. Then the delivery balloon catheter and the stent is inserted into the sheath, then during rapid pacing, and the resultant hypotension the BIB balloon is inflated (1st the inner and after a little while outer balloons), so the stent is deployed. Resultant pressure gradient drop along with residual angiographic stenosis at the site of coarctation are the determinants for post-dilatation requirement. Lesser than 30% residual stenosis along with pressure gradient lower than 20 mmHg are considered as a successful procedure. An example of a successful stent coarctoplasty is shown in Fig. 2.
Nearly all the CP stents used were 8 zig. The length of them was determined according to the length of coarctation on a diagnostic angiogram to cover about 10 mm of the normal zone and the distance of the coarctation site just after the subclavian artery branching (If covered stents would have been used). Covered stents were considered if the aorta was interrupted and in older patients whom the risk of dissection or rupture was relatively high. In the cases with consequent significant dissection or suspicious perforation after 1st uncovered stent deployment the 2nd covered stent was also deployed (Fig. 3). Balloon diameter was determined according to the diameter of the aorta just proximal to the coarctation site and in the cases with juxtaductal coarctation, the diameter of the aorta just proximal to subclavian artery was the determinant of the balloon size.
Descriptive data were gathered first. These include number of patients, age, gender, the mean duration of follow-up, procedural success rate, devices used for the procedure, acute procedural complications and follow-up data. To evaluate the association of pre-specified variables with acute and long-term results, the calculated mean and median were used if the data had a normal or non-normal distribution respectively. To compare the calculated means or medians, Chi-square, Mann-Whitney and Kruskal-Wallis tests have performed where appropriate. Variables with p<0.1 are analyzed by multivariate regression.
Of the 152 patients referred for interventional coarctoplasty, 133(87.5%) underwent successful procedures. Other 19 patients were undergone either surgical (6 patients) or balloon only coarctoplasty (13 patients). The reasons for surgical repair preference were due to wiring failure, unfavorable anatomy along with older age and presence of other significant structural heart diseases. Balloon coarctoplasty was committed in younger patients who were in the growing ages so the opportunity to put a stent at the coarctation site would be deferred until adulthood.
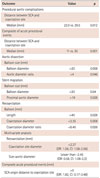
Table 3 shows the devices, which were used during coartoplasty. Routinely, we have used a long 10-14 F introducer sheath and avoided the need to pre-dilate the stenosis. We have used a bare CP stent as our routine stent. Covered CP stents were chosen for patients with interrupted aorta (11 [8.2%]) and patients with new dissection before or after stenting (2 [1.5%]) which was considered significant enough to treat with. All of the stents jailing subclavian (10 [7.5%]) artery were uncovered. None of these patients had reduced left radial pulses and clinical manifestations of hand ischemia including pain after the procedure and during follow-up.
Table 4 shows the procedural outcomes after coarctoplasty. There was no association between aortic arch morphology and any of acute procedural complications (procedural aortic complications, embolic events, stent migration). However, procedural aortic procedural complications (dissection or perforation) were more frequent in patients whom the distance between subclavian artery origin to the coarctation site was shorter {median 22 (interquartile range, [IQR]: 3.5-56) vs. 29.50 (IQR: 12-40); p=0.012}. In this point of view, composite of acute procedural events (i.e. acute embolic events, CVA, acute procedural aortic dissection, access site complications, mortality and balloon rupture) was more frequent in patients with shorter distance between SCA origin to the coarctation site rather than longer distance (median 11 [IQR 1-17.5] vs. median 30 [IQR 12-40]; p=0.001). There is also a trend (although non-significant) toward procedural aortic dissection if larger balloons were chosen rather than smaller balloon diameter (balloon diameter more than 20 mm, p=0.058), but higher balloon diameter: coarctation ratio was associated with procedural aortic dissection (p=0.046 for balloon: coarction ratio >4).
Regarding stent migration, under-sized balloons (balloon diameter under 20 mm, p=0.04) and larger aortic diameter proximal to coarctation site (aortic diameter >19, p=0.028) were associated with stent migration requiring second stent deployment. No association was found between operator's experience and acute aortic dissection or stent migration although there was a nonsignificant trend toward more frequent composite of acute events in patients whom lower experienced operators incorporated in coarctoplasty (p=0.06).
No association was found between pre-specified variables (including sheath size) and the frequency of major access site complications.
We have only 1 (0.8%) peri-procedural mortality, due to acute procedural CVA after stent coarctoplasty.
The follow-up includes clinical outpatient visits, CT-angiography, and echocardiography. There were 8 (6%) cases without follow up. Unfortunately, CT-angiography and echocardiography were not done for every patient, so there were considerable missing cases (67 [50%]). CT-angiography and echocardiography were performed for 41 (31%) and 33 (23%) respectively, and others (51 [38%]) were undergone clinical follow-up only.
Among 41 patients who were undergone CT-angiography 3 (7.3%) had an aneurysm at the site of coarctation. We found no association between aneurysm formation and related variables. All of the aneurysms were small and were treated conservatively.
As CT and echocardiography can diagnose recoarctation we had 69 valid cases. Among them 7 (10.1%) of cases had restenosis. Balloon length more than 40 (p=0.028), aorta diameter at the site of coarctation smaller than 2.35 mm (p=0.008) and balloon: coarctation diameter ratio less than 6.45 (p=0.026) was associated with higher rate of restenosis. Two out of seven of them had significant stenosis and had more than 20 mmHg gradients at the site of previous intervention (precoarctation) requiring recoarctoplasty.
Coarctoplasty can treat HTN associated with coarctation of the aorta. Comparison between the prevalence of HTN before and after coarctoplasty showed a significant reduction of HTN prevalence (117 [88%] vs. 95 [71%] p<0.001). This therapeutic effect shows a focal rather than continuous behavior during follow-up, i.e. although the prevalence reduces over time it was non-significant (95 [71%]) vs. 81 [63.8%] for HTN after coarctoplasty vs. HTN at the end of follow-up, p=0.068). Fig. 4 shows the therapeutic effect of coarctoplasty on HTN and the number of drugs after treatment. Persistent HTN after coarctoplasty was more common in older patients (age >24, p=0.013). We found no association between the presence of LVH by echocardiography and persistence of HTN at the end of follow-up.
Multivariate analysis showed no association between procedural aortic complications and pre-specified variables. These results were persistent in terms of access site complications either. However multivariate analysis showed that recoarctation is more frequent during long term follow up if the coarctation diameter was lower than 2.37 mm (OR: 1.56 with CI: 1.08-2.26) and if the smaller sized balloon has used for coarctoplasty (OR: 0.58 for balloon size-aorta diameter difference lesser than -2.45 mm with CI: 1.08-2.2). Composite acute procedural events are more frequent among patients with Juxta-ductal coarctation (i.e. the shorter distance between SCA origin and coarctation site) than postductal coarctation (i.e. the longer distance between SCA origin and coarctation site), (OR: 1.82 for Juxta-ductal coarctation, CI: 0.17-3.48) (Table 5).
Stent coarctoplasty is deemed as the treatment of choice in adult patients with native or recurrent coarctation of aorta.6) From 152 patients referred for this treatment, 133 underwent successful stent coarctoplasty, and for the remainder of the patients, the therapy was balloon only coarctoplasty or converted into surgical coarctoplasty. Most coarctoplasty procedures in our center are performed by using CP stents. To date, this stent has no FDA approval, but a prospective study (coarctation of the aorta stent trial) is ongoing to evaluate the results after coarctoplasty using this stent type.7) The conversion rate of stent coarctoplasty into surgical coarctoplasty was not recognized in previous studies, but this obviates patient's chance to undergo a lower risk procedure (i.e. stent coarctoplasty). The most important causes are a failure of passing the wire through the coarctation site, especially in interrupted aortas, and the presence of other significant structural malformations, along with older age. The practitioner operator can perform a more complex approach (e.g. using a Brocken-brought needle within small distance interrupted aorta to pass through it, which conveys the highest risk for aortic rupture) or choose surgical coarctoplasty.
Previous studies, and off course our study, demonstrated no association with the aortic arch shape and acute complications, so stent coarctoplasty can be performed safely in all forms of aortic arch shape. In regard to aortic arch shape, long-term follow-up of patients showed that persistent HTN after coarctoplasty is more prevalent in patients with gothic shaped aortic arch rather than other arch shapes8) although chronic complications such as aneurysm formation and recoarctation do not influence by aortic arch morphology.
Acute aortic complications are rare during coarctoplasty. Using an appropriate balloon size in which calculated balloon diameter: coarctation diameter ratio becomes less than 4, there is a considerably reduced likelihood of acute dissection and aneurysm formation. Another important risk factor for acute aortic complications is aortic wall stiffness. Older patients have stiffer aorta.5)9) We've chosen balloon diameter according to aortic diameter proximal to coarctation site or diameter of the aorta at the level of the diaphragm, so in 106 (72%) of our cases the calculated balloon: coarctation ratio was more than four. This obligated balloon oversizing in relation to coarctation diameter is because of the close relationship between undersized balloon (when compared to aortic diameter) and the risk of stent migration. Also, most of our patients were adults, in whom prolonged HTN may increase the stiffness of aorta. So, the relatively higher rate of acute aortic complications in our study may, to some extent, be related to the patient's age and the method of balloon sizing. Stent migration incidence is about 3.1%. The recent improvement in stent and balloon design causes a decline in the rate of stent migration.9) There are three main reasons in the Congenital Cardiovascular Interventional Study Consortium registry for stent migration: 1) Discrete fold rather than focal stenosis. 2) Balloon catheter distal displacement during inflation. 3) Balloon rupture.5) Other known risk factors may include balloon oversizing and under sizing according to aortic diameter proximal to the coarctation site.
As our database was the patient's files and minor access site complications such as minor hematoma and bleedings was not documented in them, we could not register these types of access site complications. However, in respect to major access site complications the data were documented properly. Forbes TJ et al.11) described that there is no association between sheath size and vascular injury.12) Our results also showed no association between sheath size and access site complications. Indeed, puncture site rather than the sheath size is more crucial in avoiding access site complications.
The reported mortality rate is low. In Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) registry 7 (0.7%) patients died. They have found that most of the mortalities were related to patient's comorbid conditions rather than direct procedural issues,10) however, because our patients were older, no lethal comorbid conditions were present peri-procedurally. Also, previous studies have revealed that older patients are more susceptible to CVA. We concluded that mortality causes might vary according to the patient's age.
Multivariate analysis confirmed the relationship between the SCA origin distance to coarctation site and a composite of acute procedural events. Previously our observations showed the higher probability of procedural events with shorter distance. This higher probability of procedural events is to some extent related to the proximity of coarctation site to the arch branches. In this point of view determination of the right stent/balloon size has more debate, since the proximal part of the stent should be deployed at the origin of SCA and or covered it. Therefore, stent migration or SCA coverage probability may be more common among shorter distance, i.e., juxta ductal. Of course, none of the patients whom the SCA was covered by a bare stent had ischemic upper limb signs or another morbidity, but SCA coverage is an unfavorable event. On the other hand, manipulation of the coarctation site during the intervention in shorter length patients damages the branch vessels with higher probability. The post-ductal and juxta ductal definition was considered as variables to find the impact of them on procedural events. However, this definition should be express in a different way. First, the juxta and post-ductal definition may not correct among adult patients whom their ductus arteriosus are occluded. Second, the length between SCA origin and coarctation site, as was described in the results part, is more accurate to estimate the probability of procedural complications.
The incidence of aneurysm formation varies between different studies. It has been reported as high as 17% in one study,13) although in another study with 320 cases it was 4%.14) No associations exist between aneurysm formation and recurrent coarctation, stent type and balloon size.15) Although some studies found that there might be a higher risk of aneurysm formation if balloon diameter: coarctation diameter ratio is more than 3.5:1. Most aneurysms will be treated conservatively because most are small. Predilation of the coarctation site also may predispose to aneurysm formation during long-term follow-up. So, the physician should use balloon predilation only in selected patients, which the device passage is impossible or problematic.
A younger age and over-dilation of the coarctation site (i.e. barotrauma) are predisposing factors to recoarctation/restenosis.15) We have found that using a longer balloon in length and smaller balloon in terms of diameter have an association with higher rates of restenosis. This may be because of vaster injury to the endothelium while using such a longer balloon with the larger contact area. About the smaller diameter balloons, we know that these balloons reach a high-pressure zone earlier than larger-diameter balloons during inflation according to the pressure-volume relationship, so using these balloons may cause more significant barotrauma which may cause more injury to the intima and more frequent restenosis. Of course, factors such as under dilated coarctation contributes in this observation.
Chen et al.16) demonstrated that daytime systolic blood pressure does not change immediately after coarctoplasty and there was a progressive fall in systolic blood pressure within six months. They've described that peripheral vascular dysfunction remains unchanged and may contribute to residual hypertension. In our study, different results were encountered in regard to residual hypertension after and at the end of a follow-up visit, i.e. the prevalence of HTN dropped significantly soon after coarctoplasty with modest progressive reduction of HTN prevalence over long-term follow-up. This difference may be because of the differences between the two study populations – their study included 12 patients in which 7 of them were recurrent coarctation.
In conclusion, stent coarctoplasty is a low-risk procedure with favorable early and delayed outcomes. Most mortality is related to patients' comorbid conditions and not to the procedure.
Figures and Tables
Fig. 1
Anatomical variants of the aortic arch. (A) The width and height of arch are nearly equal; it is typical for Circular arch. (B) The width is shorter than height in the typical Gothic arch. (C) Crenel arch is defined by shorter height than width. The picture beneath description of juxta and postductal coarctation. The distance between SCA origin to the coarctation site narrowing is the reference for such definition. In this case, the length is about 16 mm, which is categorized as postductal. Note the presence of localized dissection at the coarctation site with involvement of SCA origin and arch. The dissection occurred during wiring of coarctation. The covered stent was used for this patient because of dissection. SCA: subclavian artery.
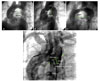
Fig. 2
Stent coarctoplasty. (A) Injection of the contrast media at descending aorta after passing the pigtail catheter over a 0.035 inch-wire. (B) Positioning of the stent. (C) Stent deployment during rapid pacing. (D) Final result.
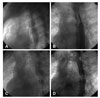
Fig. 3
Management of a patient with aortic perforation after stent deployment. (A, B) After the stent was deployed at the site of this postductal coarctation, contrast injection revealed a tiny but important contrast leakage at the distal edge of the stent. (C) A CP 38 covered stent deployed to cover the perforation site, but the leakage persisted. (D, E) Post dilation causes the leakage to stop. CP: cheatham platinum.
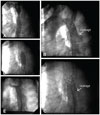
Fig. 4
Reduction in blood pressure and antihypertensive drugs number pattern after treatment of the coarctation of the aorta. (A) Systolic BP and (B) number of drugs were used for treating HTN, just after coarctoplasty and at the end of follow-up. CI: confidence interval, BP: blood pressure, HTN: hypertension.

Table 1
Baseline demographic and angiographic data

Table 2
Baseline echocardiographic characteristics of patients

Table 3
Devices used during coarctoplasty

Table 4
Procedural outcomes

Values are presented as mean±standard deviation or number (%). *Value is presented in Fig. 3. AVF: arteriovenous fistula, CVA: cerebrovascular accidents, TIA: transient ischemic attack
Table 5
Acute procedural and Follow-up outcomes and their associated variables
References
1. Peters B, Ewert P, Berger F. The role of stents in the treatment of congenital heart disease: current status and future perspectives. Ann Pediatr Cardiol. 2009; 2:3–23.
2. Baykan A, Karaqoz T, Celiker A. Endovascular stent implantation for coarctation of the aorta in children and young adults. Immediately follow-up results from Turkey. Turk J Pediatr. 2009; 51:116–119.
3. Godart F. Intravascular stenting for the treatment of coarctation of the aorta in adolescent and adult patients. Arch Cardiovasc Dis. 2011; 104:627–635.
4. Marshall AC, Perry SB, Keane JF, et al. Early results and medium-term follow-up of stent implantation for residual or recurrent aortic coarctation. Am Heart J. 2000; 139:1054–1060.
5. Holzer R, Qureshi S, Ghasemi A, Lock JE. Stenting of aortic coarctation: acute, intermediate, and long-term results of a prospective multi-institutional registry-Congenital Cardiovascular Interventional Study Consortium (CCISC). Catheter Cardiovasc Interv. 2010; 76:553–563.
6. Felts TF, Bacha E, Beekman RH 3rd, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation. 2011; 123:2607–2652.
7. Ringel RE, Gauvreau K, Moses H, Jenkins KJ. Coarctation of the aorta stent trial (COAST): study design and rationale. Am Heart J. 2012; 164:7–13.
8. Ou P, Mousseaux E, Celermajer DS, et al. Aortic arch shape deformation after coarctation surgery: effect on blood pressure response. J Thorac Cardiovasc Surg. 2006; 132:1105–1111.
9. Forbes TJ, Garekar S, Amin Z, et al. Procedural results and acute complications in stenting native and recurrent coarctation of the aorta in patients over 4 years of age: a multi-institutional study. Catheter Cardiovasc Interv. 2007; 70:276–285.
10. McCrindle BW, Jones TK, Morrow WR, et al. Acute results of balloon angioplasty of native coarctation versus recurrent aortic obstruction are equivalent. Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) registry investigators. J Am Coll Cardiol. 1996; 28:1810–1817.
11. Forbes TJ, Kim DW, Du W, et al. Comparison of surgical, stent, and balloon angioplasty treatment of native coarctation of the aorta: an observational study by the CCISC (Congenital Cardiovascular Interventional Study Consortium). J Am Coll Cardiol. 2011; 58:2664–2674.
12. Harrison DA, McLaughlin PR, Lazzam C, Connelly M, Benson LN. Endovascular stents in the management of coarctation of the aorta in the adolescent and adult: one year follow up. Heart. 2001; 85:561–566.
13. Mullen MJ. Coarctation of the aorta in adults: do we need surgeons? Heart. 2003; 89:3–5.
14. Turner DR, Gaines PA. Endovascular management of coarctation of the aorta. Semin Intervent Radiol. 2007; 24:153–166.
15. Forbes TJ, Moore P, Pedra CA, et al. Intermediate follow-up following intravascular stenting for treatment of coarctation of the aorta. Catheter Cardiovasc Interv. 2007; 70:569–577.
16. Chen SS, Donald AE, Storry C, Halcox JP, Bonhoeffer P, Deanfield JE. Impact of aortic stenting on peripheral vascular function and daytime systolic blood pressure in adult coarctation. Heart. 2008; 94:919–924.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download