Abstract
The activation of the sympathetic nervous system is associated with cardiovascular hospitalizations and death in heart failure. Renal denervation has been shown to effectively reduce sympathetic overdrive in certain patients with uncontrolled hypertension. Pilot trials investigating renal denervation as a potential treatment approach for heart failure were initiated. Heart failure comorbidities like obstructive sleep apnea, metabolic syndrome and arrhythmias could also be targets for renal denervation, because these occurrences are also mediated by the activation of the sympathetic nervous system. Therefore, renal denervation in heart failure is worthy of further investigation, although its effectiveness still has to be proven. Herein, we describe the pathophysiological rationale and the effect of renal denervation on surrogates of the heart failure syndrome.
In chronic heart failure, the activation of the sympathetic nervous system,1) the renin-angiotensin system,2) as well as pro-inflammatory activation3) are associated with remodeling processes and maladaptive cardiac signal transduction.4) Sympathetic activation plays a crucial role and is closely related to cardiovascular outcomes as judged from circulating norepinephrine concentrations.5)6) It is also likely to be involved in the progression of the syndrome because norepinephrine concentrations are already increased in asymptomatic left ventricular dysfunction before clinically relevant heart failure symptoms develop.7)
Sympathetic activation is generated by the nucleus tractus solitarius in the midbrain and rostreal ventrolateral medulla.8) Efferent signaling to the heart adapts cardiac output to peripheral stress situations with an increase of chronotropy, inotropy, and dromotropy as well as increasing intraventricular conduction velocity (bathmotropy). After longstanding activation, cardiac phenotypes can change, resulting in hypertrophy and fibrosis making the heart more prone to arrhythmia development, as well as pump function and relaxation disturbances. In heart failure, vasoconstriction and sodium retention are the result of α-adrenoceptor stimulation,9) whereas after longstanding neuroendocrine stimulation, endothelial dysfunction and oxidative stress are harbingers of structural changes of the vasculature8) and end organ damage, in particular impaired renal function.10)11) In the liver, sympathetic activation increases gluconeogenesis and glycogenolysis. Furthermore, sympathetic activation by α-adrenoceptor-mediated vasoconstriction shifts the blood flow away from insulin sensitive organs and might make patients more prone to develop impaired glucose tolerance and diabetes mellitus type 2.12)13) In the central nervous system, CO2 sensitivity is enhanced, contributing to dyspnea and conditions like heart failure,14) particularly with congestion and volume overload.15) Furthermore, sleep apnea is associated with sympathetic activation in hypertensives and patients with chronic heart failure.16)17) Blood pressure is upregulated by an increase of β1-adrenoceptor-mediated renin activation, sodium retention and an impairment of renal blood flow.8) All these conditions resemble those disturbances that are observed in chronic heart failure and in patients presenting with impaired myocardial function which is associated with a high likelihood of developing comorbidities like diabetes mellitus type 2, renal impairment and arrhythmias like atrial fibrillation or even sudden cardiac death.18) The interaction of centrally generated sympathetic drive with peripheral organs is summarized in Fig. 1.
Among these mechanisms, the interplay between renal sympathetic activity and the central nervous system is crucial.19) While activated efferent nerves from the brain increase sodium retention and reduce renal blood flow, the renal afferent nerves provide feedback to the brain with some of the signals being mediated by adenosine, oxidative stress, ischemia and acidosis.11)20)21) Afferent stimulation of the brain further increases sympathetic efferent activation leading to a vicious cycle in the interaction between brain and kidneys further enhancing total body sympathetic activity.8)11) It has been shown that sympathetic activation occurring in different forms of hypertension22) is further enhanced in heart failure,1) and in its comorbidities such as metabolic syndrome13) and renal failure.23) Thus, a sympathetic cardiorenovascular continuum occurs during the progression from mild to severe organ damage, and contributes to cardiac or renal-associated comorbidities.
Norepinephrine released from the sympathetic nerves in the heart produces excessive beta-adrenergic receptor stimulation.24) As a consequence, beta receptors are downregulated,25) and post-receptor events, such as an increase of inhibitory G-proteins,26) produce an impaired effectiveness of cAMP-dependent positive inotropic agents like beta-adrenergic agonists and phosphodiesterase type 3 inhibitors.25)26) Beyond the receptor and post receptor defects, there is a depletion of cardiac norepinephrine storing and a defect of uptake in the failing heart (Fig. 2).27)28) Serum norepinephrine concentrations are associated with mortality in chronic heart failure.5)6) In addition, not only cardiac but also renal norepinephrine spillover is increased and related to the severity of heart failure.29) Interestingly, renal spillover is also associated with poor outcomes.30) The above mentioned aspects provide the pathophysiologic background for the hypothesis that an intervention at the renal sympathetic nervous system could influence the outcome in chronic heart failure by reducing detrimental sympathetic activation.
The first clinical studies on lumbal splanchnicectomy involving sympathetic renal denervation were done in severe hypertension in the 1950's. Total paralumbar splanchnicectomy led to an increase of survival rates in patients with severe hypertension and cardiovascular disease.313233) Severe adverse effects and high mortality were observed and the method was discontinued after the development of efficient and tolerable antihypertensive drugs.
Renal sympathetic denervation was performed in patients with resistant hypertension (patients being on 3 or more drugs where one has to be a diuretic, and not achieving an optimal blood pressure control 3435363738)). The associated blood pressure reduction was not accompanied by chronotropic incompetence,39) but was able to reduce peripheral artery stiffness,40) and to reduce myocardial hypertrophy, which appeared at least partly blood pressure independent.41) These studies in hypertensives provided evidence that reducing sympathetic activity is able to reduce cardiovascular function due to reducing myocardial hypertrophy,41) thus setting the stage to conduct trials in heart failure.
Heart failure is often affected by low blood pressure hampering the possibilities for applying evidence-proven drugs often associated with further blood pressure reductions and improved outcomes. For this reason, a first pilot study (REACH, NCT 01639378) studied renal denervation in heart failure patients with reduced ejection fraction and a blood pressure above 120 mmHg systolic in an open label, uncontrolled fashion.42) Blood pressure before and after the procedure remained stable in these patients during 6 months of follow-up. Interestingly, after renal denervation there was an increase of 6-minute walk test despite no change in blood pressure. The reason might be that renal sympathetic denervation can redistribute the blood flow after reduction of sympathetic activation, and counteract the sympathetically mediated reduction of the venous reservoir and sodium water retention, thereby reducing congestion.43) However, given the uncontrolled fashion of the study, one has also to consider potential placebo effects.
Atrial fibrillation is due to functional changes of the atria, which follow the progressive remodeling of the ventricles in hypertrophy with preserved or impaired left ventricular fraction. There is a high prevalence and incidence of atrial fibrillation in heart failure which produces a symptomatic burden in these patients and also increases the risk of stroke.18) Furthermore, heart failure is associated with sleep apnea, which in turn is associated with atrial fibrillation. In an experimental study, intermittent negative tracheal pressure was associated with an enhanced inducibility of atrial fibrillation, which was accompanied by a shortening of the atrial effective refractory period.44) Renal denervation abolished the electrophysiological effect and reduced atrial fibrillation by 70%.45) The atrial fibrillation cycle length was not affected, but there was a better rate control reflected by an increase of the cycle length of the ventricle during atrial fibrillation. Trials are ongoing to study the effect of renal denervation on atrial remodeling and the recurrence rate after pulmonary vein isolation.46) Interestingly, renal denervation was associated with a reduction of atrial size as judged by echocardiography47) and magnetic resonance imaging.48) Remodeling of the atria was observed to be independent of blood pressure, but related to fewer atrial ectopies (Fig. 3).49) In a sheep model of atrial remodeling, renal denervation inhibited renal sympathetic nerve sprouting in the atria and reduced the complexity of atrial fibrillation compared with controls.50)
In a model with acute myocardial ischemia and reperfusion in pigs, renal denervation reduced ventricular ectopies and ventricular fibrillation.51) This effect was not accompanied by action potential changes and was not occurring during reperfusion showing that abolition of ventricular fibrillation during ischemia might be directly due to an effect of sympathetic withdrawal by renal denervation. There are preliminary reports on patients with cardiomyopathy suffering an electrical storm. In these patients, renal denervation on the background of full antiarrhythmic therapy and optimized heart failure treatments abolished discharges (ATPs and shocks) from an implanted ICD52) (Fig. 4). More data and larger case series have been recently presented.53)
In patients with resistant hypertension, renal denervation was safe in terms of deterioration of renal function in the Symplicity-HTN trials. However, in these trials only patients with an estimated glomerular filtration rate (GFR) >45 mL/min/1.73 m2 were enrolled. In preliminary studies it was shown that blood pressure reduction was similar in patients with impaired renal function36) or terminal renal failure.54) However, even at lower levels of GFR, there was no signal of deterioration of renal function, at least when renal denervation was performed by investigators experienced with the technique and careful use of contrast medium.36)54) However, renal denervation was able to reduce microalbuminuria, most likely by an improvement of intrarenal hemodynamics.55) In sleep apnea induced in pigs, renal denervation was able to abolish the drop in renal perfusion and attenuated the rise in renin activation after obstructive episodes.56) Data on the long term renal effects in conditions other than hypertension are presently lacking.
Patients with symptomatic heart failure suffer from insulin resistance and diabetes in 50% of the cases. Insulin resistance is depending on sympathetic activation and is most likely due to a shift of blood flow away from insulin sensitive organs.11121314) In patients with resistant hypertension, renal denervation has been shown to improve an impaired fasting glucose level. Furthermore, there was a reduction of fasting insulin and fasting C-peptide concentrations. Insulin sensitivity was improved in patients with glucose intolerance and resistant hypertension as judged from the HOMA index.57) However, it appears possible that renal denervation could provide an upstream therapy for the development of metabolic disease in situations where sympathetic activation is enhanced.
Heart failure is associated with activation of the sympathetic nervous system, which presumably results in a progression of the syndrome and thereby in poor outcome. Renal denervation should be studied in conditions with enhanced sympathetic activity. In heart failure, the first studies are ongoing assessing whether renal denervation can improve myocardial function and signs and symptoms of heart failure in patients with both preserved and reduced ejection fraction. It is necessary to study clinical outcomes in larger prospective trials involving sham procedures, because symptomatic, subjective improvements are affected by placebo and Hawthorne effects in interventional trials. Furthermore, novel interventional approaches,58) devices, and trial designs59) according to recent consensus conferences60) must be taken into consideration. Because renal denervation has recently been shown to reduce adrenergic drive in the heart,61) it is a promising approach to improve outcomes in patients with different cardiovascular diseases including chronic heart failure.
Figures and Tables
Fig. 1
Pathophysiological interaction between the brain, the kidney and other peripheral organs like heart, liver and vasculature after sympathetic activity is enhanced. Efferent signals generated in the sympathetic nervous system stimulate the heart and other organs producing maladaptive responses. In the kidney, sympathetic activation reduces renal blood flow, increases sodium retention and activates renin-angiotensin system. Efferents further enhance sympathetic outflow providing a vicious cycle in the stepwise increase of the sympathetic activation in the interaction between the heart and the brain. modified from reference 11. RAAS: renin angiotension aldosteron system.
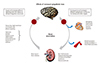
Fig. 2
Summary of beta-adrenergic signal transduction in heart failure. Modified from reference.28) AR: adrenoreceptor, NA: noradrenaline, Gs: stimulatory G protein, AC: adenylyl cyclase, Gi: inhibitory G protein, mRNA: messenger ribonucleic acid, Gia: inhibitory guanine binding protein.
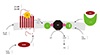
Fig. 3
Left atrial de-remodeling according to tertiles of left ventricular and left atrial volume index at baseline. Modified according to Schirmer et al.49) LAVI: left atrial volume index.

Fig. 4
Effect of renal denervation in a patient with dilated cardiomyopathy presenting with an electrical storm. Depicted are the numbers of VF episodes (left) and the systolic blood pressure values (right). It becomes clear that after one week of renal denervation no further charges of the ICD were detected. The blood pressure was particularly low in this patient, but remained stable over time. Modified according to Ukena et al.52) RDN: renal denervation, VF: ventricular fibrillation.

Acknowledgments
MB received scientific support and speakers honoraria from Bayer, Boehringer Ingelheim, Medtronic and Servier. MB is supported by the Deutsche Forschungsgemeinschaft and FM by the Hochdruckliga and the German Cardiac Society. FM received scientific support and speaker honoraria from Medtronic and SJM.
References
1. Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol. 2009; 54:375–385.
2. Holmer S, Rinne B, Eckardt KU, Le Hir M, Schricker K, Kaissling B, Riegger G, Kurtz A. Role of renal nerves for the expression of renin in adult rat kidney. Am J Physiol. 1994; 266:F738–F745.
3. Hofmann U, Frantz S. Basic How can we cure a heart “in flame”? A translational view on inflammation in heart failure. Basic Res Cardiol. 2013; 108:356.
4. Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006; 7:589–600.
5. Cohn JN, Levine TB, Olivari MT, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984; 311:819–823.
6. Rector TS, Olivari MT, Levine TB, Francis GS, Cohn JN. Predicting survival for an individual with congestive heart failure using the plasma norepinephrine concentration. Am Heart J. 1987; 114:148–152.
7. Francis GS, Benedict C, Johnstone DE, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation. 1990; 82:1724–1729.
8. Esler M. The 2009 Carl Ludwig Lecture: Pathophysiology of the human sympathetic nervous system in cardiovascular diseases: the transition from mechanisms to medical management. J Appl Physiol. 2010; 108:227–237.
9. DiBona GF, Sawin LL. Role of renal nerves in sodium retention of cirrhosis and congestive heart failure. Am J Physiol. 1991; 260:R298–R305.
10. Campese VM. Neurogenic factors and hypertension in chronic renal failure. J Nephrol. 1997; 10:184–187.
11. Böhm M, Linz D, Ukena C, Esler M, Mahfoud F. Renal denervation for the treatment of cardiovascular high risk-hypertension or beyond? Circ Res. 2014; 115:400–409.
12. Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003; 108:3097–3101.
13. Mancia G, Bousquet P, Elghozi JL, et al. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007; 25:909–920.
14. Sobotka PA, Krum H, Böhm M, Francis DP, Schlaich MP. The role of renal denervation in the treatment of heart failure. Curr Cardiol Rep. 2012; 14:285–292.
15. Dunlap ME, Sobotka PA. Fluid re-distribution rather than accumulation causes most cases of decompensated heart failure. J Am Coll Cardiol. 2013; 62:165–166.
16. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000; 342:1378–1384.
17. Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001; 19:2271–2277.
18. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012; 33:1787–1847.
19. Ljungqvist A, Wågermark J. The adrenergic innervation of intrarenal glomerular and extra-glomerular circulatory routes. Nephron. 1970; 7:218–229.
20. Stella A, Zanchetti A. Functional role of renal afferents. Physiol Rev. 1991; 71:659–682.
21. Katholi RE, Whitlow PL, Hageman GR, Woods WT. Intrarenal adenosine produces hypertension by activating the sympathetic nervous system via the renal nerves in the dog. J Hypertens. 1984; 2:349–359.
22. Esler M, Lambert G, Jennings G. Regional norepinephrine turnover in human hypertension. Clin Exp Hypertens A. 1989; Suppl 1. 75–89.
23. Hausberg M, Kosch M, Harmelink P, et al. Sympathetic nerve activity in end-stage renal disease. Circulation. 2002; 106:1974–1979.
24. Swedberg K, Viquerat C, Rouleau JL, et al. Comparison of myocardial catecholamine balance in chronic congestive heart failure and in angina pectoris without failure. Am J Cardiol. 1984; 54:783–786.
25. Böhm M, Beuckelmann D, Brown L, et al. Reduction of beta-adrenoceptor density and evaluation of positive inotropic responses in isolated, diseased human myocardium. Eur Heart J. 1988; 9:844–852.
26. Böhm M, Gierschik P, Jakobs KH, et al. Increase of Gi alpha in human hearts with dilated but not ischemic cardiomyopathy. Circulation. 1990; 82:1249–1265.
27. Goldstein DS, Brush JE Jr, Eisenhofer G, Stull R, Esler M. In vivo measurement of neuronal uptake of norepinephrine in the human heart. Circulation. 1988; 78:41–48.
28. Böhm M, La Rosée K, Schwinger RH, Erdmann E. Evidence for reduction of norepinephrine uptake sites in the failing human heart. J Am Coll Cardiol. 1995; 25:146–153.
29. Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986; 73:615–621.
30. Petersson M, Friberg P, Eisenhofer G, Lambert G, Rundqvist B. Long-term outcome in relation to renal sympathetic activity in patients with chronic heart failure. Eur Heart J. 2005; 26:906–913.
31. Page IH. The effect on renal efficiency of lowering arterial blood pressure in cases of essential hypertension and nephritis. J Clin Invest. 1934; 13:909–915.
32. Page IH, Heuer GJ. The effect of renal denervation on the level of arterial blood pressure and renal function in essential hypertension. J Clin Invest. 1935; 14:27–30.
33. Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc. 1953; 152:1501–1504.
34. Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009; 373:1275–1281.
35. Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011; 57:911–917.
36. Hering D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol. 2012; 23:1250–1257.
37. Symplicity HTN-2 Investigators. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010; 376:1903–1909.
38. Esler MD, Krum H, Schlaich M, Schmieder RE, Böhm M, Sobotka PA. Symplicity HTN-2 Investigators. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012; 126:2976–2982.
39. Ukena C, Mahfoud F, Kindermann I, et al. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol. 2011; 58:1176–1182.
40. Brandt MC, Reda S, Mahfoud F, Lenski M, Böhm M, Hoppe UC. Effects of renal sympathetic denervation on arterial stiffness and central hemodynamics in patients with resistant hypertension. J Am Coll Cardiol. 2012; 60:1956–1965.
41. Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012; 59:901–909.
42. Davies JE, Manisty CH, Petraco R, et al. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol. 2013; 162:189–192.
43. Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail. 2011; 4:669–675.
44. Linz D, Schotten U, Neuberger HR, Böhm M, Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm. 2011; 8:1436–1443.
45. Linz D, Mahfoud F, Schotten U, et al. Renal sympathetic denervation suppresses postapneic blood pressure rises and atrial fibrillation in a model for sleep apnea. Hypertension. 2012; 60:172–178.
46. Ahmed H, Miller MA, Dukkipati SR, et al. Adjunctive renal sympathetic denervation to modify hypertension as upstream therapy in the treatment of atrial fibrillation (H-FIB) study: clinical background and study design. J Cardiovasc Electrophysiol. 2013; 24:503–509.
47. Schirmer SH, Sayed MM, Reil JC, et al. Improvements in left ventricular hypertrophy and diastolic function following renal denervation: effects beyond blood pressure and heart rate reduction. J Am Coll Cardiol. 2014; 63:1916–1923.
48. Mahfoud F, Urban D, Teller D, et al. Effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: data from a multi-centre cardiovascular magnetic resonance imaging trial. Eur Heart J. 2014; 35:2224–2231.
49. Schirmer SH, Sayed MM, Reil JC, et al. Atrial remodeling following catheter-based renal denervation occurs in blood pressure- and heart rate-independent manner. JACC Cardiovasc Interv. 2015; 8:972–980.
50. Linz D, van Hunnik A, Hohl M, et al. Catheter-based renal denervation reduces atrial nerve sprouting and complexity of atrial fibrillation in goats. Circ Arrhythm Electrophysiol. 2015; 8:466–474.
51. Linz D, Wirth K, Ukena C, et al. Renal denervation suppresses ventricular arrhythmias during acute ventricular ischemia in pigs. Heart Rhythm. 2013; 10:1525–1530.
52. Ukena C, Bauer A, Mahfoud F, et al. Renal sympathetic denervation for treatment of electrical storm: first-in-man experience. Clin Res Cardiol. 2012; 101:63–67.
53. Ukena C, Mahfoud F, Ewen S, et al. Renal denervation for treatment of ventricular arrhythmias: data from an international multicenter registry. Clin Res Cardiol. 2016; 105:873–879.
54. Schlaich MP, Bart B, Hering D, et al. Feasibility of catheter-based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end-stage renal disease. Int J Cardiol. 2013; 168:2214–2220.
55. Mahfoud F, Cremers B, Janker J, et al. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension. 2012; 60:419–424.
56. Linz D, Hohl M, Nickel A, et al. Effect of renal denervation on neurohumoral activation triggering atrial fibrillation in obstructive sleep apnea. Hypertension. 2013; 62:767–774.
57. Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011; 123:1940–1946.
58. Tzafriri AR, Mahfoud F, Keating JH. Innervation patterns may limit response to endovascular renal denervation. J Am Coll Cardiol. 2014; 64:1079–1087.
59. Mahfoud F, Böhm M, Azizi M, et al. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J. 2015; 36:2219–2227.
60. Kandzari DE, Kario K, Mahfoud F, et al. The SPYRAL HTN Global Clinical Trial Program: Rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J. 2016; 171:82–91.
61. Donazzan L, Mahfoud F, Ewen S, et al. Effects of catheter-based renal denervation on cardiac sympathetic activity and innervation in patients with resistant hypertension. Clin Res Cardiol. 2016; 105:364–371.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download