Abstract
Background and Objectives
Subintimal angioplasty is a common treatment choice for chronic total occlusions (CTO) in the iliac and femoropopliteal arteries. This article describes the technical aspects and early outcomes of two different re-entry devices and comparison with manual re-entry technique.
Subjects and Methods
A retrospective review of 61 patients (re-entry group) treated with Outback or Pioneer Plus catheters was carried out. A matched cohort of patients (n=62) who underwent lower extremity interventions without the use of re-entry devices (manual re-entry group) were also analyzed (overall 123 patients were analyzed). Procedural success, procedural durations, patency estimates, ankle-brachial indices, and complications were analyzed.
Results
Sixty-one patients underwent Outback or Pioneer Plus guided subintimal recanalization. After the procedure, ankle-brachial indices significantly increased in all patients during follow-up. Primary patency for the entire cohort was 83% in the first month. When the re-entry device group was compared with manual re-entry group, no difference was found with respect to success, complication, and patencies between the two groups during follow-up. However, procedure duration and the amount of contrast agent used was significantly decreased in re-entry groups (p<0.001). Also, re-entry time was significantly decreased in Pioneer plus group according to Outback group (p<0.001)
Endovascular therapy for the treatment of aortoiliac and femoropopliteal arterial occlusive disease is considered a first-line therapy, especially for the TransAtlantic InterSociety Consensus (TASC) A-C lesions.1) A particularly challenging condition for the percutaneous transluminal angioplasty of peripheral arteries is chronic total occlusions (CTOs).23)4) CTOs are one of the primary causes of procedural failure in peripheral arterial interventions.5) However, the percutaneous recanalization of long CTOs of the aortoiliac and femoropopliteal arteries via conventional methods, using a guidewire and a support catheter, has only a moderate success rate, which depends on the lesion length, calcification, operator experience, and distal run-off vessels. Therefore, other methods that can increase the success rate of guide wire negotiation, both intraluminally and subintimally, are required.
Subintimal angioplasty, which allows the creation of a new channel in which blood can flow around a chronically occluded native vessel lumen is the preferred choice when antegrade crossing is a failure.6)7) One limitation of subintimal angioplasty is the inability to re-enter the true lumen distally. Re-entry devices were developed for this purpose.8) In this study, both the technical aspects and short-term follow-up of these two re-entry devices are described and compared with the conventional manual re-entry technique used in CTOs. The aim of our study was to determine the safety and efficacy of these devices when used with aortoiliac and femoropopliteal arterial CTOs and provide a comparative analysis between the two devices in terms of procedural success and complications.
After gaining the approval from the Institutional Review Board, a retrospective review of patients who underwent angioplasty for aortoiliac or femoropopliteal artery CTOs from April 2013 to May 2016 was performed. Patient characteristics, hospital and cath lab records, and angiograms were reviewed. Patient demographics, risk factors, indications for the procedure, procedural details, preoperative and postoperative vascular studies, and outcome data, such as first-month patency, peri-procedural complications, and mortality rates, were collected in a database for analysis. TASC II classifications of treated lesions, occlusion lengths, and re-entry distances were determined for 123 patients with angiographically documented CTOs. TASC A, B, and C patients, along with TASC D patients who were not eligible for operation, because of their comorbidities (heart failure, recent acute coronary syndrome, or stroke or being elderly and fragile) received endovascular interventions.
Subintimal angioplasty patients were divided into two main groups: the re-entry device (RD) group (n=61) and the manual re-entry (MR) group (n=62). The MR group patients were selected using TASC II classifications, lesion location, age, and gender. The patients who underwent endovascular treatment for peripheral arterial disease (PAD) without the use of a re-entry device were identified from the database and matched with the re-entry cases. Additionally, the RD group was divided into two subgroups: the Outback group (OB group) (n=30) and the Pioneer Plus group (PP group) (n=31).
The variables reviewed from the database included gender, age, comorbidities (diabetes mellitus, nicotin abuse, hypertension, hyperlipidemia, and coronary artery disease), preprocedural symptoms, indications for the intervention, Fontaine classification of PAD, TASC II classification of the index lesion, amount of radio-contrast agent, procedure, fluoroscopy, and re-entry time. Re-entry time was defined as the “time interval between re-entry failure with conventional guide wires and catheters to successful re-entry with a re-entry catheter (used only for RD-group patients) or the time interval between the first retrograde puncture attempt and successful re-entry with the retrograde method after failed antegrade attempts (for MR-group patients)”. Resting ankle-brachial indices (ABIs) were analyzed before the procedure and at the first month control. Surveillance protocols also included a duplex ultrasound (DUS) (diameter stenosis and peak velocities) one month after the procedure. Procedural success, patency estimates, ABIs, and complications were analyzed.
The procedure was performed under local anesthesia, and vascular access was obtained with an antegrade or retrograde approach via the ipsilateral or contralateral common femoral artery. If a distal retrograde approach was required, the distal posterior tibial artery (PTA) or anterior tibial artery (ATA) at the ankle level was typically selected. All patients received 5,000 IU of unfractionated heparin (UFH) intra-arterially through the vascular sheath. Routine activated clotting time (ACT) measurements were not performed. ACT was measured only if the procedure was longer than expected. In such cases, an extra UFH dosage was applied. Dual antiplatelet therapy (acetylsalyicylic acid at 100 mg/day and clopidogrel at 75 mg/day) was given to all patients for at least a month, and thereafter, antiplatelet monotherapy was continued for all patients.
The assessment of the cath lab records showed that CTOs were mostly crossed with a combination of a 0.035 inch hydrophilic stiff guidewire (Terumo glidewire, Tokyo, Japan) and a 4-French straight or angled angiographic catheter (Glidecath, Terumo, Tokyo, Japan). If the guidewire was not able to re-enter the true lumen after passage along the subintimal tract, attempts to gain access to the true lumen with catheter wire manipulations were routinely attempted. If all techniques failed, the retrograde approach method (for the MR Group) or a re-entry catheter (for the RD Group) was used to achieve successful re-entry into the true lumen.
The retrograde approach was performed with the patient in the decubitus position, and the ankle region was sterilized. Under fluoroscopic guidance, with two orthogonal planes used during fluoroscopy, vessel puncture was performed using a 21-Gauge 3 cm needle (Cook, Bloomington, IN, USA) and a 3-French pedal sheath (Cook, Bloomington, IN, USA) was placed in the target artery. Then, the CTO lesion was crossed subintimally in a retrograde fashion using a 0.035 inch hydrophilic guide wire. Thereafter, plain or drug-coated balloon angioplasty, followed by stent placement in the femoropopliteal arteries was performed and hemostasis was established via manual compression.
The 6-French Pioneer Plus catheter contains an intravascular ultrasound device in the tip and integrates with an intra-vascular ultrasound (IVUS) console (Volcano, San Diego, CA, USA) (Fig. 1A, B). The Pioneer monorail catheter is advanced to the desired re-entry point along the dissection plane via a 0.014 inch guidewire. Using chromoflow imaging, the true lumen is visualized by IVUS. By slowly rotating the catheter, the nitinol needle tip is oriented towards the true lumen and lined up at the 12-o'clock position on the ultrasonographic image (Fig. 1C). A nitinol needle is then advanced into the true lumen in a controlled fashion. The depth of the needle re-entry was adjusted via the safety ring on the catheter handle. After re-entering into the true lumen, a second exchange-length 0.014-inch guide wire is passed from the end of the catheter and the nitinol needle is retracted. The catheter is then withdrawn and the procedure is completed in the usual way.
The Outback LTD catheter (Cordis Corp, Bridgewater, NJ, USA) is also a 6-French-compatible catheter with a hollow 22-gauge needle for distal vessel re-entry with the aid of fluoroscopic imaging. Two orthogonal angiographic views are used for re-entry. An L-shaped fluoroscopic marker orientates the tip towards the target re-entry site. For exact positioning at the target re-entry site, the “T” fluoroscopic marker, combined with a 90° orthogonal view, confirms the desired alignment. Subsequently, the 22-gauge nitinol re-entry needle is placed into the distal vessel for re-entry into the true lumen. The Outback system is then retrieved, and the procedure is completed in the usual manner.
Technical success was defined as less than 30% residual stenosis within the intervened segment after the procedure. Primary patency was defined as the absence of occlusion or less than 50% stenosis during the first month of duplex ultrasonographic control, which was estimated via diameter stenosis and peak velocity calculations at the stent level. Complications (death, distal embolization, perforation, reintervention, and surgery) were also recorded after the first month.
Continuous variables were shown as mean±standard deviations or medians (minimum-maximum), and categorical data were presented as percentages. The one-sample Kolmogorov-Smirnov test was used to evaluate whether the distribution of continuous variables was normal or not. Continuous variables between the two groups were compared with a Student t-test or Mann-Whitney U-test. Categorical data were analyzed via Pearson's chi-square or Fisher's exact test where applicable. Data were analyzed using SPSS 20.0 (IBM Co., Armonk, NY, USA).
During a 37-month period from April 2013 to May 2016, 123 CTOs of iliac or femoropoliteal arteries were evaluated. A cohort of patients who underwent endovascular intervention for iliac or femoropopliteal artery CTOs and did not require re-entry devices was matched with the RD-group patients based on lesion location, TASC classification,9) gender, and age. No differences were observed between the RD-group and MR-group cohorts regarding comorbidities, including smoking, diabetes mellitus, hypertension, hyperlipidemia, and coronary artery disease (CAD). The matched cohort had a similar initial presentation to that of the RD-group cohort (Table 1, 2).
In 123 included CTOs, the true lumen could not be re-entered using standard catheter and wire techniques, so a re-entry catheter or retrograde technique via the pedal arteries was used. An Outback re-entry catheter was used in 30 (49%) cases and a Pioneer plus catheter was used in 31 (51%) cases in the RD Group. Sixty-two patients were included in the manual re-entry group.
Lesion characteristics were as follows: twenty-two common iliac artery occlusions, ten common and external iliac artery occlusions, two external iliac artery occlusions, 72 long superficial femoral artery (SFA) occlusions, and 15 femoropopliteal arterial occlusions. The mean length of the occlusions was 4.7 cm for the common iliac artery, 6.5 cm for the external iliac artery, 14.6 cm for the SFA, and 15.7 cm for the femoropopliteal arteries. Table 1 shows the clinical characteristics of the study groups, and Table 2 shows the lesion characteristics of the study groups.
The RD group included 61 CTOs in 61 patients (46 men and 15 women, mean age; 62 years). The indications for intervention in these 61 patients were as follows: critical limb ischemia in seven patients and moderate to severe claudication in 52 patients. True lumen re-entry was successful in 58 (95.0%) cases at the level of vessel reconstitution within 2 cm of the optimal angiographically defined target vessel beyond the occlusion, without compromising significant collaterals or branches. The re-entry catheter was advanced from the pedal arteries (twelve posterior and two anterior tibial arteries) in a retrograde fashion through a 6-French radial artery sheath. The total duration of re-entry catheter manipulation that was required to achieve re-entry was 10.3 minutes. The mean procedure time was 60.8 minutes, and the mean fluoroscopy time was 19.2 minutes.
Eight complications were observed in the RD group (two distal embolizations, one perforation of the external iliac artery, two reinterventions due to acute vessel occlusion, and three surgeries). Distal embolization was managed by thromboaspiration with a guiding catheter manually. When perforation was seen in the external iliac artery that was not related to the re-entry site or device, it was immediately sealed via the placement of a covered stent. Reintervention was performed because of abrupt vessel closure. Elective surgery was needed because of the failure of the procedure. It is important to emphasize that no significant bleeding occurred at the sites of true lumen re-entry needle deployment.
Fifty-nine of the 62 patients in the MR Group received successful endovascular procedures. One patient's CTO in the SFA could not be crossed antegradely or retrogradely and eventually the patient required an open bypass procedure. The total duration of re-entry catheter manipulation required to achieve re-entry was 19.2 minutes, which was significantly longer than the RD group (p<0.001). In the MR-group, the mean procedure duration was 74.6 minutes, and the mean fluoroscopy duration was 25 minutes (p<0.001).
Seven cases of complications were seen in this group (one death, one perforation, two distal embolization, two reintervention, and one surgery). The death was due to external iliac artery rupture. Although a covered stent was implanted immediately, the patient died due to hemodynamic collapse. Distal embolization was managed via local thrombolytic drug infusion. Reinterventions were performed because of subacute thrombosis of the implanted stents. Elective surgery was required because of the failure of the superficial femoral artery revascularization procedure.
In the first month, 87.5% of patients had open vessels, as assessed by palpating distal pulses, measuring ABIs, and duplex ultrasound. Patients showed mean increases of 0.25 and 0.22 in their ABIs in the RD and MR groups, respectively (p<0.05). The statistically important differences found in the RD and MR groups were procedure duration, fluoroscopy duration, re-entry time, and total volume of radio contrast agent (p<0.001) (Table 3).
After analyzing the two subgroups of RD group, it was observed that all the parameters were similar, except that re-entry time was shorter in the PP group, probably because of the use of IVUS guidance (p<0.001) (Table 4).
The management of lower-extremity arterial disease, especially chronic total occlusions, remains a major challenge.9)10)11) Both bypass surgery and endovascular intervention have been demonstrated to be effective in these patients in terms of preventing limb loss and relieving claudication-related symptoms. However, the short- and long-term results of these two treatment modalities had always been a subject of debate. Drug-eluting technologies (drug-coated balloons or drug-eluting stents) have become popular over the last decade, and the outcomes of infrainguinal endovascular interventions have become very promising. Thus, challenging lesions (TASC C-D), especially long chronic total occlusions of the lower limb, can now be treated via endovascular techniques. Typically, these techniques are used in cases of very elderly and highly comorbid patient subsets. The revascularization of chronic total occlusions of the peripheral arteries usually requires new techniques, such as “subintimal crossing” or dedicated devices, such as “re-entry systems”.
The treatment of peripheral arterial chronic occlusions frequently requires manipulation with a hydrophilic guidewire (loop technique) and a low-profile support catheter to re-enter the true lumen or adopting a retrograde vascular approach. A retrograde approach can sometimes be very challenging because distal vessels can also be affected by diffuse atherosclerosis.12) The primary limitation of this method is failure to re-enter into the true lumen with conventional tools after the subintimal crossing of the occlusions.
Both the Outback and Pioneer catheters are designed to enable safe and controlled re-entry into the true arterial lumen at a level as close to native vessel reconstitution as possible and without compromising collateral vessels. The Outback catheter is simple to use, but it is fluoroscopy-dependent. However, the Pioneer Plus is more precise because IVUS imaging is used. The cost and the availability of the IVUS platform are often the determining factors in choosing between these catheters.
Our study reviews a total of 123 patients who experienced true lumen re-entry during aortoiliac or femoropopliteal artery endovascular procedures for either critical limb ischemia or claudication. Similar to other reports, our technical success rate was 95%, and a demonstrated improvement in ABIs was seen at follow-up.13)14) One major difference from other reports is that in 14 cases, we used a re-entry system in a retrograde manner employing a 6-French radial sheath, which was placed in the pedal arteries. In other studies, the vessels chosen for this purpose were mostly distal SFAs or popliteal arteries.15)16)17) Specifically, the posterior tibial artery has sufficient vessel diameter and a straight route for a retrograde approach. During the procedure, we used intermittent intravenous nitroglycerine to prevent vasospasm. Another important point is the potential risk of conventional retrograde subintimal passage. This technique can easily push retrograde flep towards the common femoral artery, and this flep may occlude the profunda femoral artery, as we encountered in two cases in our center.
Success, first-month patency, and complication rates did not differ between the manual and re-entry device groups. However, radiation protection was better in re-entry device group because the procedure duration, fluoroscopy duration, and re-entry time were clearly shorter in re-entry device group. Additionally, using a decreased amount of radio-contrast agent in the re-entry group may be important, especially in patients experiencing chronic renal failure who have borderline renal functions.
Our study is a retrospective report with a moderate sample size, and the other major limitation of this study is the relatively short (one-month) follow-up period. The comparison of two techniques and two re-entry devices using a longer follow-up period would likely be helpful in future research. According to our results, the Pioneer Plus with IVUS guidance is a reasonable choice in all cases, especially for in-stent occlusions, because IVUS ensures direct visualization of the catheter's or wire's passage through the occluded stent. The learning curve for the use of both devices is reasonable: operators who have substantial experience with the treatment of peripheral chronic occlusions must use the device only a few times with a proctor to gain the needed expertise.
Access via the contralateral common femoral artery is the most widely described approach for re-entry catheters. If difficulty is encountered during the cross-over technique, advancing the cross-over sheath together with the re-entry catheter can be a good option. The ipsilateral antegrade common femoral artery approach can also be used easily.
In our single-center, single-operator experience, we noted that successful recanalization occurred for most of the lesions. First-month patency did not differ significantly from the manual re-entry group.18)19)20) Our short-term outcomes were also found to be similar to those of a current study published by Araki et al.21)
All of our patients underwent balloon angioplasty with plain or drug-coated balloons and the spot stenting of the re-entry sites under situation of necessity. Re-entry devices also have the potential to protect surgical landing zones (the popliteal and common femoral arteries) in terms of having shorter re-entry distances than the manual re-entry method.
In conclusion, the use of either the Outback or Pioneer re-entry catheter is a reasonable alternative to the manual re-entry technique for the management of chronic peripheral arterial occlusions involving the SFA and/or iliac arteries. These catheters are effective in achieving wire passage back to the true arterial lumen and in facilitating the successful endovascular treatment of chronic occlusions that would otherwise require surgical bypass operations. These devices may also enhance the endovascular intervention limits of TASC C and D lesions in in-operable patients with severe comorbidities. Radiation protection, shorter procedural times, and less contrast agent use are additional advantages of these dedicated devices.
Figures and Tables
Fig. 1
Reentry systems. (A) Outback catheter, (B) Pioneer Plus catheter, (C) demonstration of TL and FL via IVUS images are obtained by the transducer integrated to Pioneer plus reentry catheter. (D) Calculation of re-entry distance via fluoroscopy. TL: true lumen, FL: false lumen, IVUS: intravascular ultrasound.
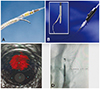
Fig. 2
Case example of RCIA stenosis and LCIA flush occlusion before (A) and after (B) the procedure with an Outback catheter. Abd Aorta: abdominal aorta, RCIA: right common iliac artery, CTO: chronic total occlusion, LCIA: left common iliac artery.
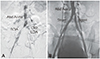
Table 1
Clinical characteristics of reentry device and manual reentry study groups

Table 2
Lesion characteristics of reentry device and manual reentry study groups

Table 3
Procedural and clinical results of study groups

Table 4
Comparison of procedural results for two reentry devices

References
1. Patel MR, Conte MS, Cutlip DE, et al. Consensus definitions for evaluation of patients with lower extremity peripheral artery disease: The Peripheral Academic Research Consortium (PARC). J Am Coll Cardiol. 2015; 65:931–941.
2. Kjellgren O, Feld S, Loyd D, et al. Successful treatment of chronic total peripheral occlusions that failed with conventional techniques using the stiff back-end of the Glidewire. Cathet Cardiovasc Diagn. 1995; 36:360–363.
3. European Stroke Organisationtion. Tendera M, Aboyans V, et al. ESC guidelines on the diagnosis and treatment of peripheral artery diseases. Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the task force on the diagnosis and treatment of peripheral artery diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011; 32:2851–2906.
4. Jaff MR, White CJ, Hiatt WR, et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the inter-society consensus for the management of peripheral arterial disease (TASC II). Ann Vasc Dis. 2015; 8:343–357.
5. Arslan S, Yuksel IO, Koklu E, et al. Clinical and morphological features of patients who underwent endovascular interventions for lower extremity arterial occlusive disease. Postepy Kardiol Interwencyjnej. 2015; 11:114–118.
6. Bolia A, Miles KA, Brennan J, Bell PR. Percutaneous transluminal angioplasty of occlusions of the femoral and popliteal arteries by subintimal dissection. Cardiovasc Intervent Radiol. 1990; 13:357–363.
7. Capek P, McLean GK, Berkowitz HD. Femoropopliteal angioplasty: factors influencing long-term success. Circulation. 1991; 83:2 Suppl. I70–I80.
8. Jacobs DL, Motaganahalli RL, Cox DE, Wittgen CM, Peterson GJ. True lumen devices facilitate subintimal angioplasty and stenting of total chronic occlusions: Initial report. J Vasc Surg. 2006; 43:1291–1296.
9. Patel SD, Biasi L, Paraskevopoulos I, et al. Comparison of angioplasty and bypass surgery for critical limb ischaemia in patients with infrapopliteal peripheral artery disease. Br J Surg. 2016; [Epub ahead of print].
10. Spanos K, Kouvelos G, Karathanos C, Xhepa S, Giannoukas A, Matsagkas M. New devices to cross chronic total occlusion in critical limb ischemia. J Cardiovasc Surg (Torino). 2016; [Epub ahead of print].
11. Hamur H, Onk OA, Vuruskan E, et al. Determinants of Chronic Total Occlusion in Patients With Peripheral Arterial Occlusive Disease. Angiology. 2016; [Epub ahead of print].
12. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007; 45:Suppl S. S5–S67.
13. Tønnesen KH, Sager P, Karle A, Henriksen L, Jørgensen B. Percutaneous transluminal angioplasty of the superficial femoral artery by retrograde catheterization via the popliteal artery. Cardiovasc Intervent Radiol. 1988; 11:127–131.
14. London NJ, Srinivasan R, Naylor AR, et al. Subintimal angioplasty of femoropopliteal artery occlusions: The long-term results. Eur J Vasc Surg. 1994; 8:148–155.
15. Laxdal E, Jenssen GL, Pedersen G, Aune S. Subintimal angioplasty as a treatment of femoropopliteal artery occlusions. Eur J Vasc Endovasc Surg. 2003; 25:578–582.
16. Yilmaz S, Sindel T, Lüleci E. Bilateral transpopliteal approach for treatment of complex SFA and iliac occlusions. Eur Radiol. 2002; 12:911–914.
17. Villas PA, Cohen G, Goyal A, Putnam SG 3rd, Ball D. The merits of percutaneous transluminal angioplasty of a superficial femoral artery stenosis via a retrograde popliteal artery approach. J Vasc Interv Radiol. 1999; 10:325–328.
18. Al-Ameri H, Shin V, Mayeda GS, et al. Peripheral chronic occlusions treated with subintimal angioplasty and a true lumen re-entry device. J Invasive Cardiol. 2009; 21:468–472.
19. Landis GS, Faries PL. New techniques and developments to treat long infrainguinal arterial occlusions: Use of reentry devices, subintimal angioplasty, and endografts. Perspect Vasc Surg Endovasc Ther. 2007; 19:285–290.
20. Jacobs DL, Cox DE, Motaganahalli R. Crossing chronic total occlusions of the iliac and femoral-popliteal vessels and the use of true lumen reentry devices. Perspect Vasc Surg Endovasc Ther. 2006; 18:31–37.
21. Araki M, Hirano K, Nakano M, et al. Two-year outcome of the self-expandable stent for chronic total occlusion of the iliac artery. Cardiovasc Interv Ther. 2014; 29:40–46.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download