Abstract
Background and Objectives
Subjects and Methods
Results
Figures and Tables
Fig. 1
Measurement of left ventricular outflow tract diameter by transthoracic and transesophageal echocardiography. (A) On transthoracic echocardiography, left ventricular outflow tract diameter (arrow) is measured in mid-systole from inner edge to inner edge just below insertion of the aortic valve leaflets. (B) On transesophageal echocardiography, left ventricular outflow tract diameter (arrow) is measured in mid-systole from inner edge to inner edge. Sinotubular junction diameter (arrow with rounded edges) is also shown.
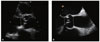
Fig. 2
TEE reclassification of patients with severe AS and normal LV ejection fraction. By transesophageal echocardiography, a significant number of patients with severe aortic stenosis are reclassified, particularly in the low flow and low flow, low gradient severe aortic stenosis groups. TTE: transthoracic echocardiography, AS: aortic stenosis, LV: left ventricle, TEE: transesophageal echocardiography.

Fig. 3
Bland-Altman plot demonstrating trend of underestimation by TTE. The wide limits of agreement between transthoracic and transesophageal echocardiography measurement likely explains reclassification of patients on the basis of left ventricular outflow tract diameter measurement. TEE: transesophageal echocardiography, LVOT: left ventricular outflow tract, TTE: transthoracic echocardiogram, SD: standard deviation.
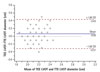
Table 1
Demographics and clinical characteristics of patients with severe aortic stenosis and normal ejection fraction by TTE

Table 2
Echocardiographic parameters in patients with severe aortic stenosis and normal ejection fraction by TTE

Data expressed as n (%) for categorical variables and mean±standard deviation for continuous variables. TTE: transthoracic echocardiogram, EF: ejection fraction, LV: left ventricle, RWT: relative wall thickness, SVI: stroke volume index, MG: mean doppler gradient, DI: dimensionless index, AVAI: aortic valve area index, TEE: transesophageal echocardiography, LVOT: left ventricular outflow tract, AV: aortic valve, ELI: energy loss index, Zva: valvuloarterial impedance
Table 3
Comparison of variability in LVOT measurement

| Coefficient of variability | |
|---|---|
| TTE intraobserver | 7.7 |
| TTE interobserver | 9.3 |
| TEE intraobserver | 9.7 |
| TEE interobserver | 9.0 |
Table 4
Clinical and echocardiographic characteristics of patients with true paradoxical low flow, low gradient severe AS versus those reclassified

Data expressed as n (%) for categorical variables and mean±standard deviation for continuous variables. AS: aortic stenosis, BMI: body mass index, BP: blood pressure, CKD: chronic kidney disease, EF: ejection fraction, LV: left ventricle, SVI: stroke volume index, MG: mean doppler gradient, DI: dimensionless index, AVAI: aortic valve area index, TTE: transthoracic echocardiography, TEE: transesophageal echocardiography, LVOT: left ventricular outflow tract, AV: aortic valve, ELI: energy loss index, Zva: valvuloarterial impedance




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download