1. van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011; 58:2241–2247.
2. King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA. 1976; 235:2506–2509.
3. McElhinney DB, Quartermain MD, Kenny D, Alboliras E, Amin Z. Relative risk factors for cardiac erosion following transcatheter closure of atrial septal defects: a case-control study. Circulation. 2016; 133:1738–1746.
4. Wang JK, Tsai SK, Lin SM, Chiu SN, Lin MT, Wu MH. Transcatheter closure of atrial septal defect without balloon sizing. Catheter Cardiovasc Interv. 2008; 71:214–221.
5. Egred M, Andron M, Albouaini K, Alahmar A, Grainger R, Morrison WL. Percutaneous closure of patent foramen ovale and atrial septal defect: procedure outcome and medium-term follow-up. J Interv Cardiol. 2007; 20:395–401.
6. Song J, Lee SY, Baek JS, Shim WS, Choi EY. Outcome of transcatheter closure of oval shaped atrial septal defect with amplatzer septal occluder. Yonsei Med J. 2013; 54:1104–1109.
7. Kim KH, Song J, Kang IS, Chang SA, Huh J, Park SW. Balloon occlusive diameter of non-circular atrial septal defects in transcatheter closure with amplatzer septal occluder. Korean Circ J. 2013; 43:681–685.
8. Du ZD, Cao QL, Rhodes J, Heitschmidt M, Hijazi ZM. Choice of device size and results of transcatheter closure of atrial septal defect using the amplatzer septal occluder. J Interv Cardiol. 2002; 15:287–292.
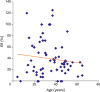
9. Helgason H, Johansson M, Söderberg B, Eriksson P. Sizing of atrial septal defects in adults. Cardiology. 2005; 104:1–5.
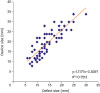
10. Zhu W, Cao QL, Rhodes J, Hijazi ZM. Measurement of atrial septal defect size: a comparative study between three-dimensional transesophageal echocardiography and the standard balloon sizing methods. Pediatr Cardiol. 2000; 21:465–469.
11. Chien JC, Hwang B, Fu YC, Lee PC, Hsieh KS, Jan SL. Sizing of atrial septal defects by intracardiac echocardiography for device closures. J Chin Med Assoc. 2008; 71:399–405.
12. Carlson KM, Justino H, O'Brien RE, et al. Transcatheter atrial septal defect closure: modified balloon sizing technique to avoid overstretching the defect and oversizing the Amplatzer septal occluder. Catheter Cardiovasc Interv. 2005; 66:390–396.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download