INTRODUCTION
MATERIALS AND METHODS
Animals and ethical statement
Isolated hindlimb model for RIC
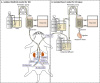 | Figure 1Schema of the perfusion system for the RIC model (A) and isolated heart model for IR injury (B). (A) After exposing the abdominal aorta, the infrarenal aorta and IVC were cannulated with 26-gauge plastic needles that were advanced to the right CIA and CIV, respectively. The aorta and IVC above the cannulated site, right CIA and right CIV above the tip of the needles, and left CIA and left CIV were tied with 5-0 silk to isolate right hindlimb circulation from systemic circulation. Heparin (200 IU) was given via venous cannulation. KHB was perfused through the arterial cannulation, and effluent from venous cannulation was collected. (B) The heart was rapidly excised via clamshell thoracotomy and placed in ice-cold KHB before being mounted on a Langendorff apparatus. KHB was used for retrograde perfusion at a constant pressure (60–80 mmHg). The KHB temperature was kept at a constant 37.0°C using a heat exchanger. A water-filled latex balloon, connected to a hydrostatic pressure transducer and coupled to a high-performance data acquisition system, was inserted into the LV and inflated to an end-diastolic pressure of 5–15 mmHg. CF was measured by timed collection of effluent over 1 minute.
CF = coronary flow; CIA = common iliac artery; CIV = common iliac vein; IR = ischemia/reperfusion; IVC = inferior vena cava; KHB = Krebs-Henseleit buffer; LV = left ventricle; RIC = remote ischemic conditioning; Rt = right.
|
 | Figure 2Design of experimental protocols. (A) RIC procedures. Anesthetized rats were subjected to either 1) a sham procedure, or 2) remote ischemia (3×5 minutes hindlimb IR using a Langendorff system). During the procedures, effluents were collected. (B) The heart from another rat was excised and perfused on a Langendorff system before being subjected to the IR protocol (30-minute regional ischemia and 60-minute reperfusion): 1) control group, 2) IPC group: prior to the IR protocol, IPC (3×1 minute LAD IR) was applied, 3) sham effluent group: effluent after sham procedure was perfused for 10 minutes before the IR protocol, and 4) RIC effluent group: effluent after RIC procedure was perfused for 10 minutes before the IR protocol. Following each Langendorff experiment, infarct size was determined using TTC staining.
IPC = ischemic preconditioning; IR = ischemia/reperfusion; LAD = left anterior descending coronary artery; RIC = remote ischemic conditioning; TTC = triphenyl-tetrazolium-chloride.
|
Isolated heart model for IR injury
Experimental protocols
Determination of area at risk (AAR) and infarct size
Immunoblotting of survival kinases
Statistical analysis
RESULTS
Effluent from the isolated hindlimb model after RIC induces significant cardioprotection
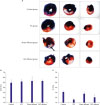 | Figure 3Representative sections of TTC-stained hearts following ischemia for 30-minute and reperfusion for 60-minute. (A) Bar graph showing the AAR expressed as a percentage of total left ventricular area (B) and IA as a percentage of AAR (C). All data is expressed as mean±SEM. n=3 in each group.
AAR = area at risk; IA = infarct area; IPC = ischemic preconditioning; RIC = remote ischemic conditioning; SEM = standard error of mean; TTC = triphenyl-tetrazolium-chloride.
*p<0.050 vs. control group.
|
Sham effluent from the isolated hindlimb model does not affect infarct size
Effluent from the isolated hindlimb model after RIC activates the SAFE pathway but not the RISK pathway
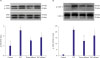 | Figure 4Western blot analysis of ERK 1/2 (A) and STAT-3 (B) phosphorylation in rat hearts subjected to ischemia-reperfusion. Top: representative immunoblots of p-ERK 1/2 and total ERK 1/2 (A) and p-STAT-3 and total STAT-3 (B) in LV homogenates from hearts subjected to ischemia-reperfusion. Bottom: bar graphs show mean±SEM of the densitometry of p-ERK to ERK ratio (A) and p-STAT-3 to STAT-3 ratio (B). Data are expressed as the mean±SEM. n=3 in each group.
AU = arbitrary units; ERK = extracellular signal-regulated kinase; IPC = ischemic preconditioning; LV = left ventricle; p-ERK = phosphorylated ERK; p-STAT-3 = phosphorylated STAT-3; RIC = remote ischemic conditioning; SEM = standard error of mean; STAT-3 = signal transducer and activation of transcription-3.
*p<0.050 vs. control, †p<0.050 vs. IPC.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download