Abstract
Background and Objectives
Subjects and Methods
Results
Figures and Tables
Fig. 1
Mean blood pressure curve during the procedure for a case of idiopathic VT under dopamine support. The patient had undergone a prior electrophysiologic study, but induced VT failed to be ablated because of hemodynamic intolerance. Instead, an ICD was implanted. Four years later, he was referred due to frequent appropriate ICD shock and underwent VT ablation. (A) It shows mean BP curve during the procedure. The blue shadow represents duration of the VT episode. External cardioversion was performed at initial VT induction due to hemodynamically unstable BP and on the fourth episode due to combined atrial fibrillation. Note that BP dropped upon initial VT induction but rose with an increase in dopamine dosage, which permitted continuous mapping and ablation. (B) Entrainment mapping at the LV low septum yielded a PPI within 20 ms of the TCL and paced QRS morphology not entirely concealed. (C) Activation mapping demonstrated the EA site (arrow) at LV septum. The EA time of pre-potential to QRS onset was -60 ms. (D) Ablation was performed at this site and terminated VT. Ablation sites were shown at (E) the right anterior oblique 35° and (F) left anterior oblique 35° views pressure. BP: blood pressure, AM: activation mapping, EM: entrainment mapping, EA: earliest activation, ABL: ablation catheter, LV: left ventricle, RV: right ventricle, PPI: postpacing interval, TCL: tachycardia cycle length, HRA: high right atrium, CS: coronary sinus, VT: ventricular tachycardia, ICD: implantable cardioverter-defibrillator, V: ventricular.
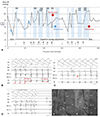
Fig. 2
Change in mean blood pressure before and after dopamine infusion. Average mean blood pressure during ventricular tachycardia of 52.3±4.1 mmHg before dopamine infusion rose to 82.6±3.8 mmHg (Δ28.8±3.2 mmHg) after dopamine infusion.

Table 1
Baseline clinical characteristics

M: male, CKD: chronic kidney disease, CVA: cerebrovascular accident, LVEF: left ventricular ejection fraction, AAD: antiarrhythmic drugs, AMD: amiodarone, ICD: implantable cardioverter-defibrillator, VT: ventricular tachycardia, LBBB: left bundle branch block, RBBB: right bundle branch block, Sup.: superior, Inf.: inferior
Table 2
Procedural characteristics and clinical outcomes

*Since case #6 was fascicular VT, successful ablation was achieved by targeting a Purkinje potential (distal to proximal), not the earliest ventricular activation site. LV: left ventricle, TCL: tachycardia cycle length, RV: right ventricle, 3D: 3-dimensional, EA: earliest activation, VT: ventricular tachycardia, L/NCC: left coronary cusp/non-coronary cusp, RVOT: right ventricular outflow tract, PM: papillary muscle




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download