Introduction
It is widely accepted that atrial fibrillation (AF) is associated with triggers originating from the muscular sleeves of the pulmonary veins.1) Therefore, pulmonary vein (PV) isolation is considered to be the cornerstone of AF catheter ablation.2) Other mechanisms involving PV, including loss of electrical coupling between the endocardial and epicardial network3)4) and pressure-related stretching,5) also play a role in the perpetuation of AF. Herein, we describe a case of PV stenosis after multiple AF catheter ablations and the association of PV stenting and AF control after the successful isolation of the PV.
Case
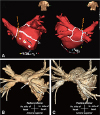
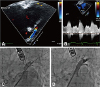
A 60-year-old man with AF was admitted to our hospital after experiencing dyspnea, orthopnea, and a non-productive cough for 2 weeks. The patient had a 10-year history of longstanding persistent AF, four previous radiofrequency catheter ablation procedures (10, 9, and 2 years, and 5 months prior to admission), and a strong family history of AF with ischemic stroke. His CHA2DS2-VASc score was 0 (male, age <60 years old, and no congestive heart failure, hypertension, diabetes, stroke/transient ischemic attack/thromboembolism, or vascular disease), and highly symptomatic paroxysmal AF remained after multiple procedures. Although this patient had undergone his 1st and 2nd procedures at another institute and we have limited information based on the medical record, he did show arrhythmogenic PV triggers from the left upper PV. At the 3rd procedure, we had found reconnected PV potentials at the left superior PV and right inferior PV and noted persistence of AF triggers from the left superior PV. Therefore, we had conducted complete circumferential PV isolation and added linear ablations. However, AF recurred as paroxysmal type. At the 4th procedure, all PV isolations and bidirectional blocks of linear ablation were maintained well, and a complex fractionated atrial electrogram-guided ablation was added (Fig. 1A). Although a left upper PV trigger occurred during the first three procedures, we did not confirm the exit block by inside PV pacing at the last procedure. To exclude potential epicardial conduction after circumferential PV isolation, inside PV pacing with maximal electrode contact might be important, especially during repeat procedures. There was no evidence of non-PV triggers after electrical cardioversion under isoproterenol infusion. At that time, 75% luminal narrowing of the left superior PV was noticed, but there were no respiratory symptoms. However, the patient became symptomatic with AF recurrence 5 months later, and 3-dimensional-computed tomography (CT) revealed progression of PV stenosis with a significantly atrophied left atrium (Fig. 1B). An increased left superior PV systolic peak flow velocity (251 cm/s) with turbulent flow at the PV ostium was observed on the transesophageal echocardiogram (Fig. 2A, B). Based on the diagnosis of clinically significant left superior PV stenosis (Fig. 2C), balloon-expandable stent deployment (10×19 mm, OMNILINK, Abbott Inc., Chicago, IL, USA) after balloon angioplasty (8×20 mm, POWERFLEX, Cordis Inc., Hialeah, FL, USA) (Fig. 2D) was performed. Sinus rhythm was restored, and the patient exhibited no apparent symptoms related to PV stenosis or AF recurrence 1 day after PV stenting. A follow-up CT performed 12 months after the procedure showed a patent left superior PV (Fig. 1C). There was no recurrence of AF based on 24-hour Holter electrocardiogram monitoring based on 2012 ACC/AHA/ESC guidelines6) during regular follow-ups for 2 years (Fig. 3).
Discussion
Considering the substantial recurrence rate of AF during long-term follow-up, repeat ablation procedures7)8) with a highly efficient irrigated tip ablation catheter may improve the clinical outcome of AF ablation.9) We targeted the left atrial antrum for circumferential PV isolation; however, high-energy repeat ablation on the PV antrum may also generate significant PV stenosis. In the current case, the patient had recurrent AF after multiple ablation procedures without evidence of PV reconnection or non-PV trigger, followed by successful control of AF by PV stenting of the left superior PV. There are several potential explanations for the antiarrhythmic effect of the PV stent. First, PV stenting could have alleviated the stretch-induced pro-arrhythmic property of the PV proximal to the stenosis. Previous reports have suggested that mechanoelectrical feedback caused by stretching regulates the arrhythmogenic activity of the atrium and PV by increasing automaticity and inducing calcium overload in the myocardial tissue.5) Conversely, relieving the pressure related to stretching of the PV by stenting may contribute to this anti-arrhythmic effect. AF was controlled immediately after the stenting, indicating that the hemodynamic burden of the lung and possibly the right heart caused the AF recurrence rather than inflammation or fibrosis. Second, there was potential epicardial conduction from the remaining PV triggers despite successful endocardial PV isolation. Pak et al.3) highlighted the importance of epicardial structures in AF ablation in cases resistant to endocardial ablation. During the repeated ablation procedure, the patient had several triggers inside the stenotic PV, and repeated RF energy delivery was required to isolate the electrical conduction of the left superior PV. Although we could not elicit the remaining PV triggers or find evidence of epicardial conduction by pacing inside PV, unrecognized PV triggers might exist. The PV stent may have induced anti-arrhythmic effects by mechanical compression, dissection, or hemodynamic change in the area of the PV triggers.
In conclusion, this is the first case report, to our knowledge, of recurrent AF with PV stenosis after multiple AF catheter ablationns successfully controlled by PV stent deployment.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download