Abstract
We report a case demonstrating a rapid and potent antiarrhythmic effect of biventricular pacing. A 67-year-old male patient with dilated cardiomyopathy was admitted for heart failure. The initial surface electrocardiogram revealed a left bundle branch block with a QRS complex duration of 200 ms. Echocardiographic examination revealed a left ventricular ejection fraction of 16%, a left ventricular end-diastolic dimension of 91 mm, and marked interventricular dyssynchrony. Continuous rhythm monitoring revealed frequently-recurring non-sustained monomorphic ventricular tachycardia (VT). Polymorphic VT, which persisted for 27 seconds, occurred on the third day after admission, and the R on T phenomenon recurred every two to three days thereafter. Optimal medical therapy for four weeks failed to suppress the recurrence of ventricular arrhythmias or ameliorate heart failure. Cardiac resynchronization therapy was initiated for an anticipated antiarrhythmic effect of biventricular pacing. Three days after the initiation of biventricular pacing, the ventricular arrhythmias disappeared almost completely.
The presence of ventricular arrhythmias in dilated cardiomyopathy patients with severely depressed left ventricular ejection fractions (LVEFs) is strongly associated with sudden cardiac arrest.1) Cardiac resynchronization therapy (CRT) may be indicated if the patients have wide QRS complexes and their left ventricular (LV) systolic dysfunction cannot be improved by appropriate medical therapy.2) Previous reports have indicated that the antiarrhythmic effect of CRT can be observed from 1 to 12 months after the initiation of biventricular pacing and is proportional to the improvement in LV systolic function.3)4) We implanted a CRT defibrillator in a dilated cardiomyopathy patient who presented with congestive heart failure and a large ventricular arrhythmia burden which had not improved following guideline-directed optimal medial therapy (OMT) for four weeks. Ventricular arrhythmias, including monomorphic and polymorphic ventricular tachycardia (VT), had disappeared almost completely three days after the initiation of biventricular pacing. This case demonstrates a rapid and potent antiarrhythmic effect of CRT.
A 67-year-old man was admitted for dyspnea of New York Heart Association Functional Classification III and generalized edema. The patient had been diagnosed with dilated cardiomyopathy five years earlier, and had recently been taking oral digoxin 0.25 mg/day, furosemide 40 mg/day, and spironolactone 25 mg/day. He had a blood pressure of 93/74 mmHg, heart rate of 80 beats per minute, respiratory rate of 26 breaths per minute, and an axillary temperature of 36.6℃. The patient reported no alcohol or substance abuse or addiction. The initial surface electrocardiogram revealed the sinus rhythm and a left bundle branch block with a QRS complex duration of 200 ms and a prolonged corrected QT interval of 550 ms (Fig. 1A). A chest X-ray revealed severe cardiomegaly (Fig. 2A). The serum digoxin level was 0.82 ng/mL (reference range: 0.8-2.0 ng/mL). The cardiac troponin-I level was 0.04 ng/mL (reference range: 0-0.04 ng/mL) and the brain natriuretic peptide level was 2351 pg/mL (reference range: 0-60 pg/mL). The serum K+ concentration was 4.0 mmol/L (reference range: 3.5-5.5 mmol/L).
Echocardiographic examinations demonstrated globally hypokinetic LV wall motion with an LVEF of 16% and LV end-diastolic dimension (LVEDD) of 91 mm. Doppler studies revealed severe functional mitral valve regurgitation and marked interventricular dyssynchrony. The LVEF measured by a magnetic resonance imaging study was 12%, and delayed gadolinium enhancements were noted along the mid-to-basal septal segments. Initial 24-hour ambulatory electrocardiographic monitoring revealed multiple episodes of nonsustained monomorphic VT, which persisted for 3-5 seconds and recurred more than 100 times. The burden of ventricular arrhythmic events was 17.8% of the total daily beats (Fig. 3A). The patient was considered to be at high risk of sudden arrhythmic death.
Because the patient had not been taking a beta blocker and an angiotensin converting enzyme or angiotensin II receptor blocker within 3 months of admission, oral bisoprolol 2.5 mg/day and perindopril 4 mg/day were added to the medication list to meet the requirements of guideline-directed OMT. Furosemide was titrated up to 80 mg/day and administered intravenously to control dyspnea and generalized edema. However, on the fifth day after admission, polymorphic VT, which was sustained for 27 seconds and accompanied by a loss of consciousness, occurred early in the morning (Fig. 3B). Although the sustained polymorphic VT terminated spontaneously, the R on T phenomenon recurred every two to three days thereafter. Class III antiarrhythmic agents such as amiodarone or sotalol were not administered due to the potential risk of prolongation of the corrected QT interval, which can cause the recurrence of fatal polymorphic VT. Although low-dose dobutamine was additionally administered intravenously at an infusion rate of 2.5 µg/min for 10 days to suppress the occurrence of ventricular arrhythmias by rapidly improving LV systolic function, the dobutamine therapy did not effectively control either the ventricular arrhythmias or heart failure. Despite guideline-directed OMT for 4 weeks, non-sustained monomorphic VT recurred dozens of times per day, and the burden of ventricular arrhythmic events at this stage was still approximately 19% (Fig. 3C). Follow-up echocardiography did not reveal significant improvement of the LVEF or reduction of the LVEDD. The patient was still considered to be at high risk of sudden arrhythmic death.
When the clinical response to the OMT for four weeks was considered, it was anticipated that medical therapy alone would fail to prevent the deterioration of heart failure and the occurrence of sudden arrhythmic death. Because we were not sure that we could eradicate or reduce the burden of ventricular arrhythmias enough to prevent sudden arrhythmic death by radiofrequency catheter ablation without procedure-related complications, we did not perform radiofrequency ablation. Although the Korean Health Insurance Review and Assessment Service guidelines recommend OMT for at least three months before the initiation of CRT, we decided to perform early CRT, with the expectation that biventricular pacing might induce rapid electrical and mechanical remodeling of the LV and suppress the recurrence of ventricular arrhythmias.
A CRT defibrillator device with a unique MultiPoint™ pacing function (Quartet™ LV lead, Durata™ right ventricular lead, Tendril MRI™ right atrial lead, Quadra Assura MP™ generator, St. Jude Medical, St. Paul, MN, USA) was implanted without complications. The electrocardiograms acquired immediately after the initiation of biventricular pacing revealed marked shortening of the QRS complex duration (from 200 to 160 ms) and of the corrected QT interval (from 550 to 500 ms) (Fig. 1B). Disappearance of the characteristic electrocardiographic findings of left bundle branch block was noted, together with a significant reduction in the ventricular arrhythmia burden from the day after the initiation of CRT. Although there was only a mild decrease in the cardiacthoracic ratio on the follow-up chest X-ray image (Fig. 2B), the burden of ventricular arrhythmic events was reduced to 0.2%, and non-sustained monomorphic VT had disappeared almost completely by the third day (Fig. 3D). Follow-up echocardiography performed on the day after CRT device implantation revealed remarkable improvements in interventricular dyssynchrony and functional mitral valve regurgitation, although the LVEF and LVEDD were not notably changed. Dyspnea and generalized edema began to improve after 3-4 days. After we confirmed the absence of recurrence of significant ventricular arrhythmias (including the R on T phenomenon), the patient was discharged without procedure-related complications 2 weeks later. Improvement of the LVEF from 20 to 24% and reduction of the LVEDD from 91 to 76 mm were documented in follow-up echocardiographic examinations nine weeks after the initiation of CRT. At a follow-up duration of 10 months, no recurrence of significant ventricular arrhythmia requiring defibrillation therapy was observed.
The most common causes of cardiac death in patients with dilated cardiomyopathy are uncompensated heart failure and fatal ventricular arrhythmias. In this patient with significantly advanced dilated cardiomyopathy, it appears that a severely dilated LV with high wall stress provided a basis for the recurrence of various ventricular arrhythmias of differing mechanisms, including delayed afterdepolarization, re-entry, and enhanced automaticity. Implantable cardioverter-defibrillator (ICD) therapy is recommended for the prevention of sudden arrhythmic death in high-risk patients for whom OMT is ineffective. However, ICD therapy does not elicit an antiarrhythmic effect in itself. In contrast, CRT produces an antiarrhythmic effect through mechanical remodeling with reduced LV wall stress, electrical remodeling with suppression of anisotropic reentry, and the reduction of circulating plasma catecholamine levels.4)5)6)7) A prior study indicated that the ventricular arrhythmia burden could be reduced if an ICD were upgraded to a CRT device, which further supports the existence of an antiarrhythmic effect of biventricular pacing.8) Previous studies have demonstrated that an antiarrhythmic effect of CRT can be observed from 1 to 12 months after the initiation of biventricular pacing, proportional to the improvement of LV systolic function.3)4) However, in this case, the suppression of ventricular arrhythmia recurrence was observed from the day after the initiation of biventricular pacing, and nearcomplete suppression was achieved in 3 days (Fig. 3). We postulate that the immediate correction of interventricular dyssynchrony and the subsequent reduction of functional mitral valve regurgitation by CRT resulted in an electrophysiologically significant reduction of LV wall stress and counteracted the increased activity of intraventricular ectopic foci. We also postulate that the remarkable shortening of the corrected QT interval from 550 to 500 ms and the reduction of intraventricular repolarization heterogeneity (made possible by biventricular pacing using the unique LV lead [Quartet™], which can pace multiple points) may have helped to suppress the R on T phenomenon recurrence and polymorphic VT. However, some animal and human case studies have raised concerns about proarrhythmic effects of biventricular pacing.9) Reversed electrical activation of the ventricles by apical and epicardial pacing may induce prolongation of the QT interval and increase the transmural dispersion of ventricular repolarization, which can be important triggers for the development of polymorphic VT.9) In contrast, another report indicated that biventricular pacing was not associated with increased incidence of polymorphic VT.10) In a practical sense, although biventricular pacing may have proarrhythmic effects to some extent in susceptible patients, it seems that the incidence of biventricular-pacing-induced polymorphic VT is not considerably high. It is also possible that biventricular-pacing-induced inhibition of sympathetic nervous activity could suppress the abnormally increased activity of intraventricular ectopic foci.11)
Recently updated guidelines recommend OMT, including renin-angiotensin-aldosterone system blocking agents and beta blockers, for at least 3 months before ICD implantation for the primary prevention of sudden arrhythmic death in ischemic or non-ischemic cardiomyopathy patients with depressed LVEFs.2)12) The guidelines also recommend CRT as a class-I indication if the LVEF is below 35% and the QRS complex duration is persistently over 150 ms with a left bundle branch block despite appropriate medical therapy. However, the guidelines do not define an appropriate duration of OMT before the initiation of CRT. In contrast, the Korean Health Insurance Review and Assessment Service guidelines recommend OMT for at least three months prior to CRT device implantation. However, if heart failure deteriorates and ventricular arrhythmias recur frequently, as in this case, early CRT is more desirable than awaiting the clinical response to medical therapy. ICD therapy alone cannot treat heart failure and prevent the recurrence of ventricular arrhythmias. If effective antiarrhythmic agents are not available or are contraindicated, a risk for repeated appropriate shock therapy exists. Recurrent ICD shock therapy, whether appropriate or not, may induce significant myocardial injury, which may further promote the occurrence of ventricular arrhythmias13)14) and cause systolic function to deteriorate in the long term.15)16) In addition, upgrading ICD to CRT 3 months later (after confirmation of medical treatment failure) according to the Korean insurance guidelines may increase the risks for delayed treatment and the occurrence of procedure-related complications. Therefore, an early start for CRT should be considered if a patient has uncompensated heart failure with a high risk of sudden arrhythmic death. However, based on the limited observations of this case, we cannot exclude the possibility that the early emergence of the antiarrhythmic effect of biventricular pacing was a merely coincidental finding. It is possible that the OMT performed for four weeks before CRT was beginning to exhibit its effects. Therefore, an early start for CRT should be considered carefully only when the patient's condition will not allow waiting for the clinical response to the standard therapies.
In summary, we treated a dilated cardiomyopathy patient with uncompensated heart failure and a high ventricular arrhythmia burden by CRT. The large ventricular arrhythmia burden was reduced rapidly as soon as biventricular pacing began. The clinical course of this case suggests that starting CRT early may be beneficial in selected patients with uncompensated heart failure and high ventricular arrhythmia burdens.
Figures and Tables
Fig. 1
Electrocardiographic findings before and after initiation of cardiac resynchronization therapy. (A) Initial electrocardiogram exhibits the sinus rhythm and notched broad QRS complexes with left bundle branch block configuration. The QRS complex duration was 200 ms, and the corrected QT interval calculated by Bazett's formula was 550 ms. (B) Follow-up electrocardiogram acquired during biventricular pacing exhibits shortening of the QRS complex duration from 200 to 160 ms and of the corrected QT interval from 550 to 500 ms. aVR: augmented voltage right arm, aVL: augmented voltage left arm, aVF: augmented voltage left foot.
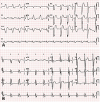
Fig. 2
Radiographic findings. (A) Initial chest X-ray displays cardiomegaly with a cardiac-thoracic ratio of 0.7 and minor fissural effusion. (B) Follow-up chest X-ray acquired 3 days after the initiation of cardiac resynchronization therapy displays a slight decrease in the cardiacthoracic ratio compared to the initial image.
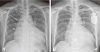
Fig. 3
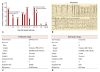
Ventricular arrhythmia burden before and after initiation of cardiac resynchronization therapy. (A) The bar graph displays the daily or average (asterisk) burdens of ventricular arrhythmias, which were recorded by 24-hour or 72-hour ambulatory electrocardiographic monitoring according to the hospital admission days. (B) Data from 24-hour ambulatory electrocardiographic monitoring acquired 5 days after admission demonstrate the occurrence of polymorphic ventricular tachycardia, which persisted for 27 seconds and terminated spontaneously. (C) Analysis of the follow-up 24-hour ambulatory electrocardiographic monitoring data acquired one day before the initiation of cardiac resynchronization therapy displays a large burden of VPCs with multiple episodes of non-sustained monomorphic VT. Although optimal medical therapy was performed for over 4 weeks, the daily burden of ventricular arrhythmias was still 19% of the total daily beats. (D) Analysis of the follow-up 24-hour ambulatory electrocardiographic monitoring data acquired 3 days after the initiation of cardiac resynchronization therapy displays near complete disappearance of VPCs and VTs. The ventricular arrhythmia burden was reduced to 0.2% of the total daily beats. VT: ventricular tachycardia, VPC: ventricular premature complex, BPM: beat per minute, VE: ventricular ectopy, PVC: premature ventricular complex.
References
1. Meinertz T, Hofmann T, Kasper W, et al. Significance of ventricular arrhythmias in idiopathic dilated cardiomyopathy. Am J Cardiol. 1984; 53:902–907.
2. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016; 18:891–975.
3. Kies P, Bax JJ, Molhoek SG, et al. Effect of left ventricular remodeling after cardiac resynchronization therapy on frequency of ventricular arrhythmias. Am J Cardiol. 2004; 94:130–132.
4. Di Biase L, Gasparini M, Lunati M, et al. Antiarrhythmic effect of reverse ventricular remodeling induced by cardiac resynchronization therapy: the InSync ICD (Implantable Cardioverter-Defibrillator) Italian Registry. J Am Coll Cardiol. 2008; 52:1442–1449.
5. de Bakker JM, van Capelle FJ, Janse MJ, et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988; 77:589–606.
6. Hamdan MH, Zagrodzky JD, Joglar JA, et al. Biventricular pacing decreases sympathetic activity compared with right ventricular pacing in patients with depressed ejection fraction. Circulation. 2000; 102:1027–1032.
7. Kies P, Bax JJ, Molhoek SG, et al. Effect of cardiac resynchronization therapy on inducibility of ventricular tachyarrhythmias in cardiac arrest survivors with either ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2005; 95:1111–1114.
8. Ermis C, Seutter R, Zhu AX, et al. Impact of upgrade to cardiac resynchronization therapy on ventricular arrhythmia frequency in patients with implantable cardioverter-defibrillators. J Am Coll Cardiol. 2005; 46:2258–2263.
9. Fish JM, Brugada J, Antzelevitch C. Potential proarrhythmic effects of biventricular pacing. J Am Coll Cardiol. 2005; 46:2340–2347.
10. McSwain RL, Schwartz RA, DeLurgio DB, Mera FV, Langberg JJ, León AR. The impact of cardiac resynchronization therapy on ventricular tachycardia/fibrillation: an analysis from the combined Contak-CD and InSync-ICD studies. J Cardiovasc Electrophysiol. 2005; 16:1168–1171.
11. Cha YM, Chareonthaitawee P, Dong YX, et al. Cardiac sympathetic reserve and response to cardiac resynchronization therapy. Circ Heart Fail. 2011; 4:339–344.
12. Russo AM, Stainback RF, Bailey SR, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Heart Rhythm. 2013; 10:e11–e58.
13. Pinski SL, Fahy GJ. The proarrhythmic potential of implantable cardioverter-defibrillators. Circulation. 1995; 92:1651–1664.
14. Runsio M, Kallner A, Kallner G, Rosenqvist M, Bergfeldt L. Myocardial injury after electrical therapy for cardiac arrhythmias assessed by troponin-T release. Am J Cardiol. 1997; 79:1241–1245.
15. Goldenberg I, Moss AJ, Hall WJ, et al. Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the multicenter automatic defibrillator implantation trial II. Circulation. 2006; 113:2810–2817.
16. Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008; 359:1009–1017.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download