Abstract
Background and Objectives
While the off-label use of implantable medical devices for treating congenital heart disease is not uncommon, the present conditions and outcomes of their off-label use have rarely been described. Therefore, this study evaluated the prevalence and results of using implantable devices off-label to treat congenital heart disease at a single institute.
Subjects and Methods
This was a retrospective study based on the medical records of catheter-based interventions for congenital heart disease performed from July 1, 1995 to June 1, 2015. The inclusion criterion was the off-label use of an implantable device. Patient demographic data, procedural success, and follow-up status regarding late complications were investigated, and the results of the off-label use of each device were compared.
Results
Off-label use occurred in 144 of 1730 interventions with device implantation, accounting for 8.3% of the interventions. The median patient age and mean body weight were 51.0 months and 16.3 kg, respectively. Immediate and late failures were found in 9 cases, and 3 cases of mortality were not directly related to the devices used. The overall success rate was 93.8%. There were no long-term complications of the off-label use of occlusion devices. No procedural failures resulted from stent implantation, but one case of stent malposition and two cases of stent fracture were identified after procedure completion.
Catheter-based cardiac intervention has become an alternative method for treating congenital heart disease (CHD) and routinely produces good outcomes. However, the relatively small patient population and wide variety of specific lesions make it more difficult to invest in this field than in that of adults with acquired heart disease. The off-label use of medications and medical devices is common in the context of pediatric disease.1)2) The increased prevalence of catheter intervention in CHD has increased the off-label use of approved devices (i.e., the use of the devices in ways other than their intended applications). Although it is possible to amend device labeling to add other approved uses, this process is often expensive and time-consuming. To our knowledge, there have been few investigations regarding the off-label use of implantable medical devices for treating CHD.2)3) The most commonly used implantable medical devices in CHD are stents and occlusion devices, such as the Amplatzer duct occluder (ADO), coils, and the Amplatzer vascular plug (AVP). The off-label use of implantable devices could be crucial because choosing on-label devices is empirically difficult due to various lesions in CHD. Therefore, we investigated the present conditions and outcomes of the off-label use of implantable transcatheter devices to treat CHD.
We reviewed all medical records of transcatheter interventions for CHD performed at Samsung Medical Center over a period of approximately 20 years from July 1, 1995 to June 1, 2015. Each transcatheter intervention for CHD was carried out by one of three pediatric interventionists at our institute. The implanted devices were classified as either occlusion devices or stents.
We defined off-label use as the use of an approved medical device for an intervention other than any of the indications mentioned in the user manual.4) We did not consider patient age as a factor of on-label use because most of the device manuals did not mention age-specific uses in pediatric patients. However, we regarded balloon-expandable stent implantation in the great vessels as on-label use. While balloon-expandable stent implantation has not yet been approved for treating pulmonary artery stenosis or coarctation of the aorta, it is traditionally regarded by interventionists as an effective and safe procedure for stenotic lesions in the great vessels. Nevertheless, the use of self-expandable stents for treating CHD and balloon-expandable stents outside of the great vessels is still defined as an off-label application. Patent foramen ovale (PFO) closure with an Amplatzer PFO device was classified as on-label use even though the Food and Drug Administration withdrew its approval for this procedure in the United States (US). The on-label uses for each device included in our study are presented in Table 1.
Our review included patient demographic data, procedural success, and follow-up status regarding late complications. Procedural failure was defined as re-intervention or operation during or after a procedure, and late complications included mechanical failures with or without hemodynamic derangement that required intervention. We investigated the proportion of both the off-label use and the failure rates for each intervention and also for each device. The use of different types of devices for different lesions in one patient was considered a multiple-device implantation, but multiple coils implanted for the same lesion were considered one coil. The data were analyzed using standard descriptive statistics, and Fisher's exact test was used to compare the success rates of the various procedures.
This study was approved by the Samsung Medical Hospital Institutional Review Board.
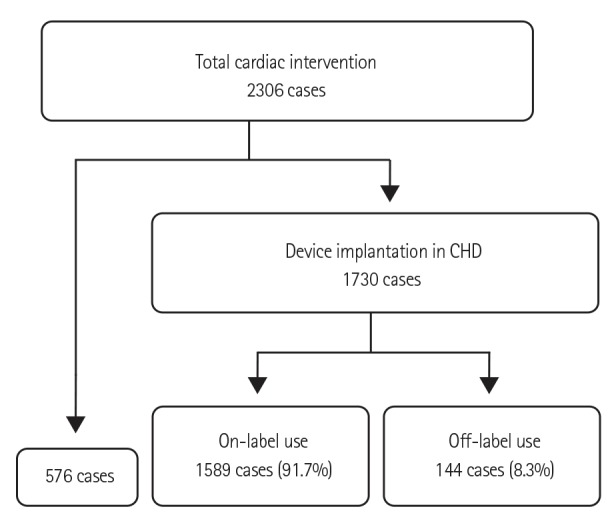
During the study period, 2306 transcatheter cardiac interventions for CHD and 1730 interventions with device implantation were performed. Off-label medical device use occurred in 8.3% (144/1730) of the interventions (Fig. 1). Of these patients, 50% (72) were male. The median patient age was 51.0 (0.5-1032.0) months (Fig. 2), and the median body weight was 16.3 (2.7-80.0) kg. Immediate and late failures from off-label use were identified in 9 cases (6.3%); 7 operations and 2 re-interventions were performed in these patients. There were 10 minor and transient complications, such as arrhythmia, thrombus formation, and embolization, related to the procedures. Three cases of mortality not directly related to the device were also detected.
Total patent ductus arteriosus (PDA) occlusion was performed in 714 cases, and off-label use accounted for 5.46% (39) of these procedures. There was no off-label device use for atrial septal defect closure. However, every case in which a device was used for Blalock-Taussig (B-T) shunt occlusion (9), banding site occlusion (2), or fenestration occlusion at the Fontan pathway (47) was off-label by definition. There were 3 muscular ventricular septal defect (VSD) closures, 6 membranous VSD closures, and 2 Gerbode shunts. All of the VSD closures performed during the investigative period were considered cases of off-label device use because the muscular VSD device was not used and the membranous VSD device was not available. Off-label device use for PFO closure accounted for 22.6% (12/53) of those cases. Of the 184 various unclassified occlusions found, 7 off-label uses were identified (Table 2).
AVPs and embolization coils were used for PDA closure in 24 (61.5%) and 15 (38.5%) cases, respectively. The ADO (10, 83.3%), the AVP (1, 8.3%), and the Amplatzer septal occluder (ASO) (1, 8.3%) were employed off-label for VSD closure; 7 ASOs, 4 CardioSEALs, and 1 StarFlex occluder were used off-label for PFO closure. In this study, AVPs (2), ADOs (2), and coils (3) were selected for the off-label treatment of various unclassified lesions, i.e., left ventricular pseudoaneurysm occlusion, coronary fistula occlusion, major aortopulmonary collateral artery embolization, occlusion of a fistula between the aorta and the right atrium, and vertical vein occlusion.
The overall success rate of the off-label use was 93.8%. The clinical success rates of the off-label use of occlusion devices were 100%, except for PDA occlusion (94.9%) and VSD closure (66.7%) (Table 3). VSD closure had a significantly lower success rate than that of PDA occlusion (p=0.022) (Table 4). There were 4 procedural failures in VSD closure due to a procedural difficulty (1), a residual lesion (1), an atrioventricular AV block during the procedure (1), and device embolization (1). Two procedural failures were found for PDA occlusion due to device embolization and device malposition, respectively (Table 5). There were no long-term complications following the off-label use of these occlusion devices.
The off-label use of stents for PDA and at other sites was 100% and 36%, respectively (Table 2). While stents used at sites other than PDA showed lower success rates than those used for PDA occlusion, the difference was not significant (p=0.078) (Table 3, 4). Three coronary stents and 13 peripheral stents (consisting of 9 balloon-expandable stents and 4 self-expandable stents) were used. There were no procedural failures from stent implantation, but one case of stent malposition and two cases of stent fracture were detected after the procedure. All three cases of stent complications were associated with the off-label use of balloon-expandable stents for treating right ventricular outflow tract (RVOT) stenosis (Table 5). All of these patients underwent surgical removal of the broken stent and RVOT reconstruction. In contrast, the off-label use of self-expandable stents for treating coarctation of the aorta (1), peripheral pulmonary artery stenosis (2), or RVOT stenosis (1) resulted in good outcomes.
This study found that 8.3% of implantable medical device interventions for CHD were off-label and had acceptable clinical outcomes. Sutherell et al.2) reported that the frequency of off-label medical device use in pediatric cardiology in the US was 63%. The primary difference identified in our investigation was that our study was limited to implantable devices and excluded stent implantations in the pulmonary artery and PFO closures performed with the Amplatzer PFO device. Another study reported that 3.9% of cases using the Amplatzer device involved off-label indications.3) Therefore, different inclusion criteria prevent a precise comparison of our results with those of other studies. However, it is within reason to presume that the off-label use of medical devices for treating CHD is not unusual.5) The wide variety of CHD and the various potential complications require interventionists to design solutions with a high degree of flexibility. In this context, we regard the off-label use of medical devices for treating CHD as “creative implementations, adaptations and modifications,” as stated by McElhinney.6) Expanding the indications of each device is a time-consuming and expensive process due to the rare conditions for which they can be used and is therefore nearly impossible. Although the off-label use of a device is not typically covered by Korean health insurance, the results of insurance inspection were not uniform in our cases. Nevertheless, it is important to evaluate the safety and efficacy of each off-label use of medical devices and highly recommended to upgrade post-market medical device surveillance systems to oversee and evaluate devices with the potential for broader market applications.5)6)
Most PDA occlusions were performed with the on-label use of the ADO. This study found that PDA occlusion was carried out with the off-label use of the AVP and coils. Gianturco coils were frequently and safely used before the era of the ADO.7) The use of multiple coils for PDA occlusion was included as an off-label use, and the safety and success of this application have been previously demonstrated.8) In addition, successful cases of PDA closure within the AVP have been reported.9)10)11)12) Therefore, along with our excellent results, these findings indicate that PDA closure with various off-label devices might not be considered unusual.
Most VSD closures were performed with the off-label use of the ADO. Good immediate results of membranous VSD closure and muscular VSD closure with the ADO have been previously reported.13)14) While the success rate of VSD closure with the off-label use of this device was significantly lower than the others, we should consider that the study period included the early period of VSD closure implementation in our country. In the past, membranous VSD closure was not a popular procedure in our country, and it was very rare for muscular VSDs to be closed using a device. The reasons for failure were not serious, and all VSDs in these cases were closed completely by surgery or re-intervention. The off-label use of the ADO for VSD was demonstrated to be safe and effective over the long-term in our study. For some patients with large PFOs, the off-label use of the Amplatzer septal occluder was applied with great success.15) Because off-label use regulations might vary in different countries, we regarded the use of the Amplatzer PFO device for PFO closure as an on-label application. Other PFO closure devices were not available in South Korea during the study period. Very rare conditions, such as left ventricular pseudoaneurysm and coronary fistula, were previously reported to have been closed successfully with the ADO or AVP.3) In our study, no off-label occlusion devices produced problems after successful implantation. However, the technical or mechanical aspects of occlusion devices used off-label should be evaluated cautiously.
Although the first stents were placed within various lesions, including peripheral pulmonary arteries, in children with CHD in 1991,16) almost all stents including PDA are used off-label in CHD.17)18) However, we acknowledge stent use at the peripheral pulmonary artery as traditionally on-label because we sought common reasons for using devices off-label in CHD. Due to the limited amount of material available in our country, the off-label uses of typically approved stents in unusual areas were reviewed. While good results were obtained in our patients after PDA stenting, significantly lower success rates resulted from RVOT stenting. All but one of these failures was associated with stent fracture and the use of balloon-expandable stents. The stent fractures were easily observed for the RVOT, and the rate of fracture has been reported to range from 25-40%.19)20)21) However, there were no late complications from self-expandable stents, even in the RVOT. Despite our very limited experience treating congenital heart disease with self-expandable stents, we again emphasize the superiority of the off-label use of self-expandable stents compared with balloon-expandable stents in the RVOT.
This study had various limitations. It was a retrospective study conducted by a single institution, and it therefore cannot represent the current status of all patients in our country. The indications of the off-label use of implantable medical devices were not generally recognized but instead were determined largely based on operator'spreference. Therefore, their frequency could differ widely across studies. Nevertheless, these findings demonstrate that there is a great need to amend the clinical indications of implantable medical devices for treating CHD. We believe that this study provides significant information to facilitate the expansion of clinical applications for the off-label use of medical devices.
In conclusion, the off-label use of implantable devices for treating CHD was found to be safe and effective in specific situations. The off-label use of balloon-expandable stents in RVOT should be performed more cautiously than that of other implantable devices.
References
1. Holzer RJ, Sisk M, Phillips A. Hybrid balloon pulmonary valvuloplasty in a 700-g infant: thinking outside the box. Catheter Cardiovasc Interv. 2008; 72:93–96. PMID: 18383180.
2. Sutherell JS, Hirsch R, Beekman RH 3rd. Pediatric interventional cardiology in the United States is dependent on the off-label use of medical devices. Congenit Heart Dis. 2010; 5:2–7. PMID: 20136851.
3. Baspinar O, Irdem A, Kilinc M. Off-label use of Amplatzer devices in congenital heart disorders during childhood. Acta Cardiol. 2013; 68:31–35. PMID: 23457907.
4. David Y, Hyman WA. Issues associated with off label use of medical devices. Conf Proc IEEE Eng Med Biol Soc. 2007; 2007:3556–3558. PMID: 18002765.
5. Holzer R, Hijazi Z. The off-versus on-label use of medical devices in interventional cardiovascular medicine?: clarifying the ambiguity between regulatory labeling and clinical decision making, part III: structural heart disease interventions. Catheter Cardiovasc Interv. 2008; 72:848–852. PMID: 19006259.
6. McElhinney DB. Beyond indications: postmarket surveillance and the importance of expanded and off-label use of transcatheter devices in structural and congenital interventions. Circ Cardiovasc Interv. 2012; 5:739–740. PMID: 23250968.
7. Moore JW, Khan M. Gianturco coil occlusion of patent ductus arteriosus. Curr Interv Cardiol Rep. 2001; 3:80–85. PMID: 11177723.
8. Zellers TM, Wylie KD, Moake L. Transcatheter coil occlusion of the small patent ductus arteriosus (<4 mm): improved results with a “multiple coil-no residual shunt” strategy. Catheter Cardiovasc Interv. 2000; 49:307–313. PMID: 10700064.
9. Cho EH, Song J, Kang IS, et al. Transcatheter closure of small ductus arteriosus with amplatzer vascular plug. Korean J Pediatr. 2013; 56:396–400. PMID: 24223601.
10. Cho YK, Chang NK, Ma JS. Successful transcatheter closure of a large patent ductus venosus with the Amplatzer vascular plug II. Pediatr Cardiol. 2009; 30:540–542. PMID: 19294462.
11. Delaney JW, Fletcher SE. Patent ductus arteriosus closure using the Amplatzer® vascular plug II for all anatomic variants. Catheter Cardiovasc Interv. 2013; 81:820–824. PMID: 23074167.
12. Ng B, Schneider DJ, Hokanson JS. Closure of tubular patent ductus arteriosus in infants and small children with the Amplatzer Vascular Plug II. Congenit Heart Dis. 2011; 6:64–69. PMID: 21269416.
13. Kim SJ, Huh J, Song JY, Yang JH, Jun TG, Kang IS. The hybrid perventricular closure of apical muscular ventricular septal defect with Amplatzer duct occluder. Korean J Pediatr. 2013; 56:176–181. PMID: 23646056.
14. Lee SM, Song JY, Choi JY, et al. Transcatheter closure of perimembranous ventricular septal defect using Amplatzer ductal occluder. Catheter Cardiovasc Interv. 2013; 82:1141–1146. PMID: 23554093.
15. Walsh KP, Wilmshurst PT, Morrison WL. Transcatheter closure of patent foramen ovale using the Amplatzer septal occluder to prevent recurrence of neurological decompression illness in divers. Heart. 1999; 81:257–261. PMID: 10026348.
16. O'Laughlin MP, Perry SB, Lock JE, Mullins CE. Use of endovascular stents in congenital heart disease. Circulation. 1991; 83:1923–1939. PMID: 2040045.
17. Hascoet S, Baruteau A, Jalal Z, et al. Stents in paediatric and adult congenital interventional cardiac catheterization. Arch Cardiovasc Dis. 2014; 107:462–475. PMID: 25128078.
18. Lee KE, Seo YJ, Kim GB, et al. Complications of cardiac catheterization in structural heart disease. Korean Circ J. 2016; 46:246–255. PMID: 27014356.
19. Breinholt JP, Nugent AW, Law MA, Justino H, Mullins CE, Ing FF. Stent fractures in congenital heart disease. Catheter Cardiovasc Interv. 2008; 72:977–982. PMID: 19021285.
20. Peng LF, McElhinney DB, Nugent AW, et al. Endovascular stenting of obstructed right ventricle-to-pulmonary artery conduits: a 15-year experience. Circulation. 2006; 113:2598–2605. PMID: 16735676.
21. Powell AJ, Lock JE, Keane JF, Perry SB. Prolongation of RV-PA conduit life span by percutaneous stent implantation. Intermediate-term results. Circulation. 1995; 92:3282–3288. PMID: 7586315.
Fig. 2
The distribution of patient age at the time of transcatheter intervention with medical devices used off-label.
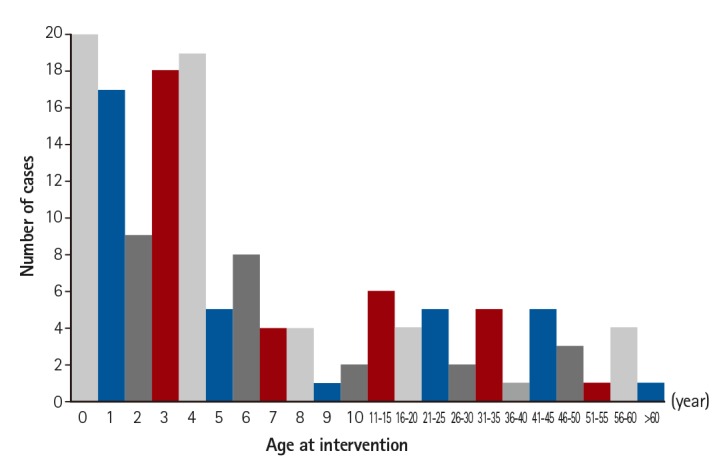
Table 1
On-label use for each device as indicated by the FDA-approved device labeling
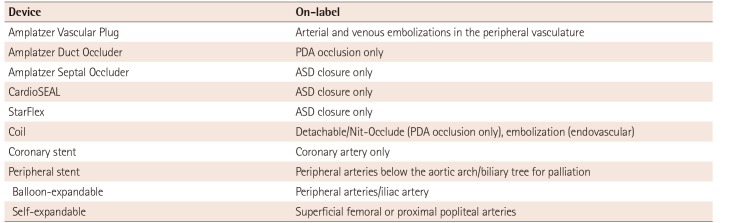
Table 2
The proportion of off-label uses in transcatheter interventions for congenital heart disease with device implantation
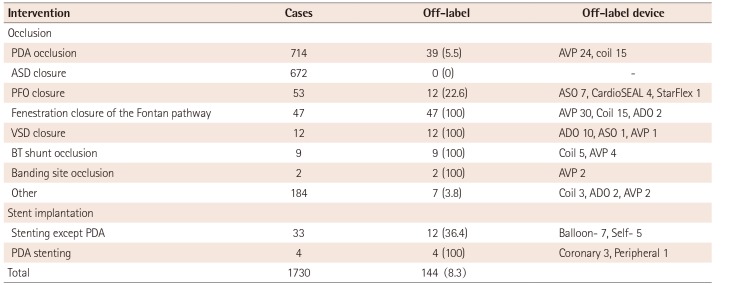
Table 3
Clinical success rates of the off-label use for each procedure and a comparison of each procedure
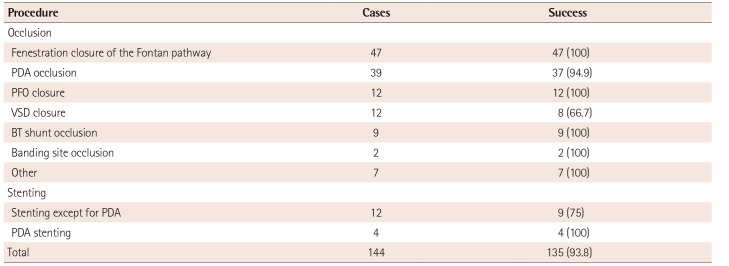
Table 4
Clinical success rates of the off-label use of each device
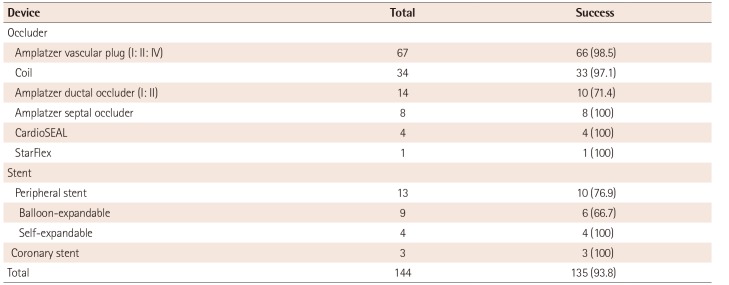
Table 5
Procedural failures either during or after a procedure
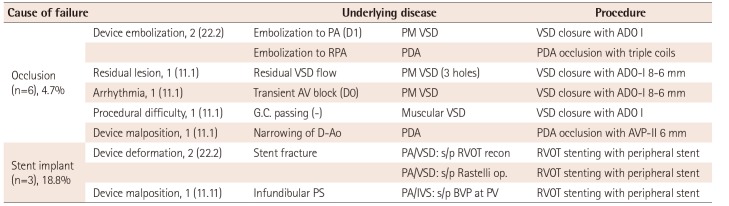
Values are presented as number (%). PA: pulmonary artery, D: day, PM: perimembranous, VSD: ventricular septal defect, ADO: amplatzer duct occluder, RPA: right pulmonary artery, PDA: patent ductus arteriosus, AV: atrioventricular, D-Ao: descending aorta, AVP: amplatzer vascular plug, PA/VSD: pulmonary atresia with ventricular septal defect, PS: pulmonary stenosis, PA/IVS: pulmonary atresia and intact ventricular septum, BVP: balloon valvuloplasty, PV: pulmonary valve, RVOT: right ventricular outflow tract




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download