Abstract
Background and Objectives
We studied the results of patient management for left isomerism (LI) and sought to determine factors that may influence survival and prognosis.
Subjects and Methods
We reviewed the medical records of 76 patients who were compatible with LI criteria between 1982 and 2014.
Results
Of the total study population, 29 patients (38.1%) had functional univentricular heart disease, 43 patients (56.5%) had cardiac anomalies suitable for biventricular hearts, and four patients (5.2%) had normal heart structure. Extracardiac anomalies were noted in 38.1% of the study population, including biliary atresia in 7.8% of all patients. Of the 25 patients who underwent Kawashima procedures, 24.0% developed pulmonary arteriovenous fistulas (PAVFs). During the median follow-up period of 11.4 years (range: 1 day to 32 years), 14 patients died. The 10-year, 20-year, and 30-year survival rates were 87%, 84%, and 76%, respectively. Preoperative dysrhythmia and uncorrected atrioventricular valve regurgitation were significantly associated with late death. There was no significant difference in the number of surgical procedures and in survival expectancy between patients in the functional single-ventricle group and in the biventricular group. However, late mortality was higher in functional single-ventricle patients after 18 years of age.
Heterotaxy syndrome, including right atrial isomerism (RI) and left atrial isomerism (LI), encompasses a variety of cardiovascular and visceral anomalies. Cardiac malformations are a major component of heterotaxy syndrome, resulting in significant morbidity and mortality. LI involves bilateral left atrial appendages, polysplenia, and a wide range of cardiac malformations. Patients with LI or polysplenia syndrome frequently share characteristics of either RI or asplenia syndrome, and the complexity of cardiac malformation is often less severe in LI than in RI. However, extracardiac conditions are sometimes more serious in LI, and, to date, the long-term overall outcomes of LI have not been well documented.
Accordingly, we conducted a retrospective study of LI to evaluate its clinical characteristics and long-term outcomes. We also sought to determine the factors that may influence survival and long-term prognosis in patients with LI.
We searched the databases of the Department of Pediatrics and the Department of Cardiovascular Surgery at Seoul National University Children's Hospital for cases of LI or isomerism between 1982 and 2014. This study was approved by the Institutional Review Board of Seoul National University Hospital. Patients were considered for inclusion following our review of their medical records, chest X-rays, computed tomography scans, magnetic resonance imaging, electrocardiography, echocardiography, cardiac catheterization with angiography, and abdominal sonography. We confirmed the diagnosis of LI if the patient satisfied one or more of the following criteria: (1) morphologically bilateral left atrial appendage (long and narrow attachment to the atrium body), (2) situs ambiguous with bilateral hyperarterial bronchi or bilobed lung, and (3) interrupted inferior vena cava (IVC) with azygous continuation, together with at least one other feature of LI, including sinus node dysfunction, partial anomalous pulmonary venous return, common atrium, atrioventricular septal defect (AVSD), congenital atrioventricular (AV) block, biliary atresia, polysplenia, or malrotation of the gut.
Two patients who lacked spleens were excluded, as they could potentially be mixed phenotypes.
We defined heart failure by a composite of left ventricular ejection fraction ≤40%, a history of taking inotropes for heart failure, and medical records indicating dyspnea at rest or with minimal exertion. We confirmed liver cirrhosis by abdominal sonography, computed tomography scans, or magnetic resonance imaging. In terms of AV valve regurgitation, descriptions of more than moderate degree or three-over-four grade regurgitation were categorized as significant AV regurgitation.
Medical records containing reports of surgical procedures, catheterizations, noninvasive imaging, and clinical follow-up were reviewed.
Categorical variables were analyzed using Fisher's exact tests. Survival was estimated in accordance with the method of Kaplan and Meier, and the differences between the groups were analyzed using log-rank and Wilcoxon tests. Variables that were related to mortality or morbidity with p<0.15 in a univariate analysis were entered into a multivariate model with stepwise forward selection and an entry criterion of p<0.05. All statistical analyses were performed using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA).
A total of 76 patients, including 39 females and 37 males, were enrolled for final analysis in this study. The age of presentation to the hospital ranged from birth to 11 years of age. With 47 neonatal patients (61.8%), the patients most frequently presented as neonates, followed by 17 patients (22.4%) presenting at one to twelve months of age, and 12 patients (15.8%) presenting at twelve months of age or older. With 27 patients (35.5%), LI diagnosis was most frequently made during the fetal period. The latest diagnosis was made in one patient at 24 years of age.
Anatomical distributions and primary diagnosis of cardiac anomalies are summarized in Table 1. There were 29 patients (38.1%) with functional univentricular heart disease that required Fontan type palliations, 43 patients (56.5%) with cardiac anomalies suitable for biventricular hearts, and four patients who, except for isolated IVC interruption, had normal cardiac structure.
Sixty-four (84.2%) patients underwent heart surgeries. The median age at the time of initial heart surgery was 10.5 months (range: 1 day to 25 years). Twenty-nine patients required interim surgical palliative procedures based on the combination of malformations prior to the ultimate surgery.
Out of the 29 patients with functional univentricular hearts, Fontan operations were performed in 23 patients (79.3%). The type of Fontan pathways were lateral tunnels in 13 patients (56.5%), aortopulmonary collaterals (APCs) in five patients, and extracardiac pathways in five patients. Fenestration of the Fontan pathways was performed in nine cases (39.1%). Twenty-six patients underwent bidirectional cavopulmonary shunt, which included the Kawashima procedure in 25 cases, at a median age of 3.0 years (range: 0.5 years to 18.5 years). Twenty-two out of the 25 patients in the Kawashima group underwent a Fontan completion at a median age of 4.9 years (range: 1.2 years to 18.5 years).
Fig. 1 depicts the clinical courses and surgical data of the patients with left isomerism. Additional surgery, before or after the final step of the surgical programs-either biventricular or univentricular-was necessary in 35 patients (48.6%). In the group of patients receiving biventricular repairs, 22 patients (51.1%) required additional surgeries in a total of 32 cases. In the group of patients that received univentricular repairs, 13 patients (44.8%) required additional surgeries in a total of 18 cases.
The mean number of total surgical procedures (2.1±1.1 biventricular repairs vs. 2.2±1.0 single-ventricle surgeries; p=0.60) was not significantly different between the biventricular repair group and the single-ventricle surgery group (Table 2). Fig. 2 depicts the estimated proportion of patients free from additional surgeries, showing 43 patients with hearts suitable for biventricular repairs and 29 patients with hearts suitable for single-ventricle surgeries. Additional surgeries were performed more frequently in the biventricular repair group (p=0.02).
A total of 18 patients underwent AV valve surgeries. Nine patients had significant AV valve regurgitation at the time of initial echocardiography. Five of the nine patients with significant AV valve regurgitation underwent valve surgeries. Of the 18 patients that underwent AV valve surgeries, 13 patients developed new AV valve regurgitation during the postoperative follow-up period. Only two of the 18 patients that underwent AV valve surgeries had significant AV valve regurgitation according to their most recent echocardiography.
Overall, artificial valve replacement was performed in a total 17 cases comprising 10 patients. In the 10 patients, the artificial valve replacement procedures involved common atrioventricular valves in five cases, the mitral valve in three cases, the tricuspid valve in three cases, the pulmonary valve in three cases, the aortic valve in two cases, and the mitral valve together with the aortic valve in one case.
At presentation or during follow-up before the operation, dysrhythmias were observed in 14 patients (18.4%). During the whole follow-up period, cardiac rhythm abnormality became common. There were 55 patients (72.4%) with dysrhythmia at the end of the follow-up period. Abnormalities of cardiac rhythm are summarized in Table 3. Sinus node dysfunction developed in 36 patients (47.3%), including junctional rhythm in 19 patients (25.0%) and sinus pause in seven patients. Of these, 12 patients who had sinus node dysfunction with bradycardia were found at presentation or prior to surgery. Atrioventricular block (AV block) was also frequently found. Complete AV block occurred spontaneously in five patients and post-surgically in three patients. Pacemaker implantation was performed in 23 patients (30.2%), at the mean age of 9.6±9.8 years (range: 1 day to 30 years). Out of these 23 patients, 14 patients (60.8%) received pacemaker implantation for sinus node dysfunction, and five patients received pacemaker implantation for AV block. In four patients, a pacemaker was implanted concomitantly with Maze procedures for atrial fibrillation. Two patients with cardiac anomalies required urgent pacemaker implantation for sinus node dysfunction during the first week of life.
The initial mode of the pacemaker was DDD(R) in 13 patients, AAI(R) in seven patients, and VVI(R) in three patients. Multivariate analysis showed there was no independent factor requiring pacemaker implantation among the patients with pacemakers. The risk for bradycardia requiring a pacemaker was not related to type of cardiac anomaly (p=0.822), surgical modality (p=0.071), or extracardiac disease (p=0.051). There were 34 patients (44.7%) with an unusual P axis, which was not a significant factor associated with the need for pacemaker implantation or with sinus node dysfunction.
Polysplenia was confirmed in 39 patients (79.6%), and a single normal spleen was observed in 10 patients (20.4%). Extracardiac disease and abnormalities among the patients are summarized in Table 4. Extracardiac diseases were associated in 29 patients (38.1%). In terms of hemato-oncologic diseases, there were two cases of pheochromocytoma, one case of a small round cell tumor of the patient's tibia, and one case of acute leukemia. Surgical treatment was performed in three patients with hepatobiliary disease, and in eight patients with gastrointestinal disease. One Fontan-palliated patient underwent successful liver transplantation at three years of age.1)
The median duration of the follow-up period was 11.4 years (range: 1 day to 32 years). With respect to late morbidity, out of the 76 patients with LI, there were twelve patients with heart failure, seven patients with PAVFs, nine patients with liver cirrhosis, eleven patients with hepatic congestion at sonography, five patients with splenomegaly, and three patients with ascites. Eleven patients had taken percutaneous interventions for progressive cardiovascular lesions.
Patients who had developed PAVFs showed progressive hypoxemia, which was confirmed by contrast saline echocardiography, computed tomography scans, or pulmonary angiography. Of the 25 patients who underwent Kawashima procedures, manifest PAVFs occurred in six (24.0%) patients at a median age of 11.4 years (range: 4.0 years to 21.2 years) following the Kawashima procedures. The PAVFs developed at the ipsilateral side of azygous drainage (Table 5). Five of the PAVF patients had undergone multiple coil embolization procedures for persistent PAVFs at a median age of 18.3 years (range: 12.4 years to 27.5 years). After the coil embolization procedures among the patients, the patient mean systemic arterial saturation (SaO2) in room air immediately increased from 79.5±3.8% (range: 75% to 84%) to 91.0±2.7% (range: 89% to 95%). At the time of the most recent follow-up, the patient mean SaO2 in room air was 89.0±2.6% (range: 86% to 91%). In 76 patients, the possibility of a Kawashima procedure was the risk factor associated with PAVFs when analyzed by binary logistic regression (OR 15.7, 95% CI; 1.7-139.9). Patients with PAVFs showed a risk of heart failure (OR 2.0, 95% CI; 0.3-11.7), liver cirrhosis (OR 1.3, 95% CI; 0.2-9.2), and hemato-oncologic disease (OR 1.6, 95% CI; 0.1-21.7).
A total of 61 cardiopulmonary exercise tests were performed in 32 (42.1%) LI patients at a median age of 21 years (range: 8 years to 36 years). The median maximal oxygen consumption (VO2 max) was 29.2 mL/kg/min (range: 11.1 mL/kg/min to 42.7 mL/kg/min). Among the patients that underwent cardiopulmonary exercise tests, there were 16 patients with biventricular repairs and 16 patients with single-ventricle repairs. There was no significant difference in the mean VO2 max between the two groups.
The development of heart failure was associated with patients having a functional univentricular heart (OR 6.6, 95% CI; 1.6-27.0), patients that underwent Fontan operations (OR 6.5, 95% CI; 1.7-24.7), patients that underwent Kawashima procedures (OR 5.5, 95% CI; 1.4–20.7), and patients with liver cirrhosis (OR 10.7, 95% CI; 2.3–49.4). The burden of ventricle pacing among patients with a pacemaker, uncorrected significant AV valve regurgitation, extracardiac disease, or PAVFs did not differ between those with or without heart failure (Table 6).
In comparing late outcomes between the groups of patients with biventricular repairs and single-ventricle surgeries, patients with single-ventricle surgeries showed a risk of catheter intervention (OR 8.5, 95% CI; 1.6–43.6), heart failure (OR 8.5, 95% CI; 1.6-43.6), liver cirrhosis (OR 5.9, 95% CI; 1.1–31.4), and PAVFs (Table 2).
There were 14 cases of patient mortality. Five (8.1%) out of 61 patients who underwent cardiac surgery died within 30 days during the postoperative period. Four out of five early postoperative deaths occurred at a median age of two months (range: 1 month to 12 months), following Blalock-Taussig shunt procedures in two cases, and following pulmonary artery banding and other procedures in two cases. The causes of death included unstable hemodynamics, pulmonary hypertensive crisis, and respiratory failure due to respiratory viral infection. Three out of five early postoperative deaths occurred prior to 1998. Nine cases of mortality were characterized by late or nonsurgical death at a median age of 19 years (range: 10 days to 40 years), which represents a median of 6.5 years following the final surgery in patients. The presumptive causes of late death were acute renal failure related to Fontan failure in two patients, malignant pheochromocytoma in one patient, and hepatobiliary disease with intractable gastrointestinal bleeding in one patient. Two patients had unknown sudden death, and there were two patients with nonsurgical death. Eight out of 14 patients (57.1%) expired at the hospital.
The cumulative survival rates were 87% in 10 years, 84% in 20 years, and 76% in 30 years. The mean survival between the functional univentricular heart group and the group of others was not significantly different (30.4 years vs. 36.1 years, p=0.23). Fig. 3 depicts the estimates of survival for 76 patients with normal hearts, with hearts suitable for biventricular repair, and with hearts suitable for single-ventricle surgery. The late mortality rate was higher in the group of single-ventricle surgery patients than in the group of patients with biventricular repairs when patient age was over 18 years (66.6% vs. 0.0% respectively; p<0.05).
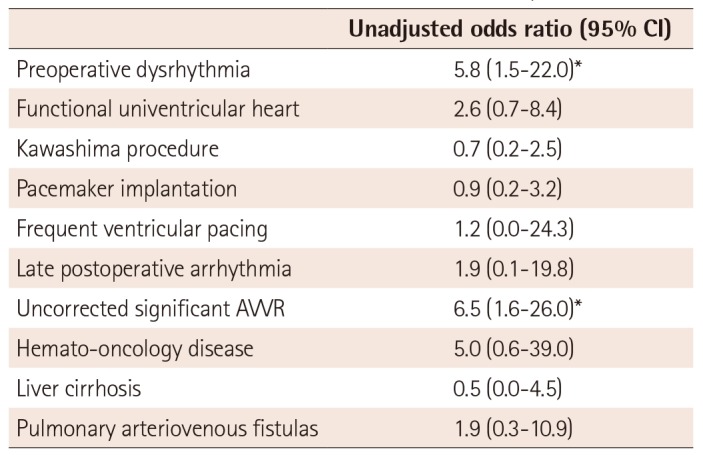
Univariate analysis showed functional univentricular hearts, preoperative dysrhythmia, and uncorrected significant AV valve regurgitation were associated with mortality (p<0.15). Multivariate regression analysis showed that with an entry criterion of p<0.05, preoperative dysrhythmia and uncorrected significant AV valve regurgitation were significant risk factors (Table 7).
This study demonstrates the spectrum of cardiac and extracardiac manifestations in LI. Additionally, this study presents risk factors associated with morbidity and mortality, as well as the overall outcomes.
AVSD or common AV valves are frequent occurrences in heterotaxy syndrome. A recent study2) reports that AV valve regurgitation and anomalous pulmonary venous connection are considered to be the most important contributors to increased mortality in heterotaxy syndrome with a single ventricle. In our experience, 55.3% of LI patients had a common AV valve, and most of them received AV valve surgery at least once. Instead of the excellent long-term outcomes observed in this subgroup, uncorrected AV valve regurgitation is considered a significant risk factor for reinterventions and mortality.
In LI, sinus nodes may not be detected under a light microscope in serial sections, or sinus nodes may be abnormally hypoplastic and situated laterally in the atrium.3)4) It has been proposed that a decrease in sinus node cells or an increase in fibrosis of the atrial wall is a common phenomenon in sinus node dysfunction and slow atrial rhythms with LI. This may manifest as sinus pause, sinus bradycardia, or sick sinus syndrome by dysfunction of the sinus nodes. Some reports have demonstrated that atrial rhythm with an axis between -30° and -90° is the most frequent rhythm in LI.5)6) As reported in a previous study,7) many patients exhibit progressive slowing of the heart rate, varying degrees of AV block, or supraventricular tachyarrhythmia. Overall, pacemaker implantation was required in 30.2% of LI patients in our study. Of the total patients, 44.7% had an unusual P axis, which was not a significant factor associated with pacemaker implantation or sinus node dysfunction.
The estimated frequency of primary ciliary dyskinesia (PCD), also called immotile-cilia syndrome, is 1 in 12000 to 1 in 20000 live births. Pheochromocytoma is a very rare disease in childhood, usually occurring in the setting of a hereditary syndrome in 1 in 36000 individuals. We encountered a high overall incidence of PCD (2.6%) and pheochromocytoma (2.6%) in patients with LI.
It is widely assumed that PAVFs develop due to the exclusion of hepatic venous blood from pulmonary circulation following Kawashima operations.8)9) However, after hepatic vein inclusion or redirection, PAVFs may persist in heterotaxy syndrome with interrupted IVC.10) Recent studies10)11) have documented that there is an association between the persistence of PAVFs and a lateralized flow stream from the systemic venous to pulmonary circulation, such as the conduit position on the side contralateral to the superior vena cava (SVC) with azygous drainage, or on the side with a solitary SVC without interrupted IVC. In our study, all patients had PAVFs that were unilateral in the ipsilateral side of azygous drainage.
In recent reports,2)12) survival rates following Fontan procedures in heterotaxy and non-heterotaxy patients have been comparable. Moreover, recent advances in cardiac surgery may offer improved prospects for survival in patients with complex heart disease. In our study, the overall survival time for LI, which was estimated using the Kaplan-Meier method, was 33.3 years. Any deaths that occurred during intensive care unit stays or within 30 days of cardiac surgery were reported as surgical mortalities. In our study, the total surgical mortality of 8.3% is comparable to previously published data. This report also compares the long-term outcomes of biventricular repairs and single ventricle repairs. Although the number of surgical procedures and the rate of early postoperative mortality were not significantly different between the groups, Fontan-palliated patients showed a higher rate of late heart failure and late hepatic complications, as well as a tendency for higher late mortality, than other patients.
In conclusion, it is necessary to understand a wide range of manifestations and to closely monitor LI patients not only for dysrhythmia, valve regurgitation, and the development of PAVFs, but also for noncardiac systemic manifestations.
References
1. Youn JK, Lee JM, Yi NJ, et al. Pediatric split liver transplantation after Fontan procedure in left isomerism combined with biliary atresia: a case report. Pediatr Transplant. 2014; 18:E274–E279. PMID: 25263970.
2. Smith A, McKay R. Atrial arrangement(situs)/Isomerism/Juxtaposition. A practical atlas of congenital heart disease. 1st ed. London: Springer-Verlag London Ltd.;2004. p. 41–52.
3. Stamm C, Friehs I, Duebener LF, et al. Improving results of the modified Fontan operation in patients with heterotaxy syndrome. Ann Thorac Surg. 2002; 74:1967–1977. discussion 1978. PMID: 12643382.
4. Ho SY, Seo JW, Brown NA, Cook AC, Fagg NL, Anderson RH. Morphology of the sinus node in human and mouse hearts with isomerism of the atrial appendages. Br Heart J. 1995; 74:437–442. PMID: 7488461.
5. Momma K, Takao A, Shibata T. Characteristics and natural history of abnormal atrial rhythms in left isomerism. Am J Cardiol. 1990; 65:231–236. PMID: 2296892.
6. Wren C, Macartney FJ, Deanfield JE. Cardiac rhythm in atrial isomerism. Am J Cardiol. 1987; 59:1156–1158. PMID: 3578058.
7. Frogoudaki A, Sutton R, Gatzoulis MA. Pacing for adult patients with left atrial isomerism: efficacy and technical considerations. Europace. 2003; 5:189–193. PMID: 12633645.
8. Hashmi A, Abu-Sulaiman R, McCrindle BW, Smallhorn JF, Williams WG, Freedom RM. Management and outcomes of right atrial isomerism: a 26-year experience. J Am Coll Cardiol. 1998; 31:1120–1126. PMID: 9562017.
9. Uemura H, Ho SY, Devine WA, Kilpatrick LL, Anderson RH. Atrial appendages and venoatrial connections in hearts from patients with visceral heterotaxy. Ann Thorac Surg. 1995; 60:561–569. PMID: 7677481.
10. Kim SJ, Bae EJ, Lee JY, Lim HG, Lee C, Lee CH. Inclusion of hepatic venous drainage in patients with pulmonary arteriovenous fistulas. Ann Thorac Surg. 2009; 87:548–553. PMID: 19161777.
11. Kim SJ. Heterotaxy syndrome. Korean Circ J. 2011; 41:227–232. PMID: 21731561.
12. Takeuchi K, Murakami A, Hirata Y, Kitahori K, Doi Y, Takamoto S. Surgical outcome of heterotaxy syndrome in a single institution. Asian Cardiovasc Thorac Ann. 2006; 14:489–494. PMID: 17130325.
Fig. 2
Kaplan-Meier freedom from additional surgeries curves for 72 patients with left isomerism and hearts suitable for biventricular repair (n=43) and hearts suitable for single-ventricle surgery (n=29). Differences between groups were analyzed using log-rank and Wilcoxon tests.

Fig. 3
Kaplan-Meier survival curves for 76 patients with left isomerism and normal heart structure (n=4), with hearts suitable for biventricular repair (n=43), and with hearts suitable for single-ventricle surgery (n=29). Differences between groups were analyzed using log-rank and Wilcoxon tests.
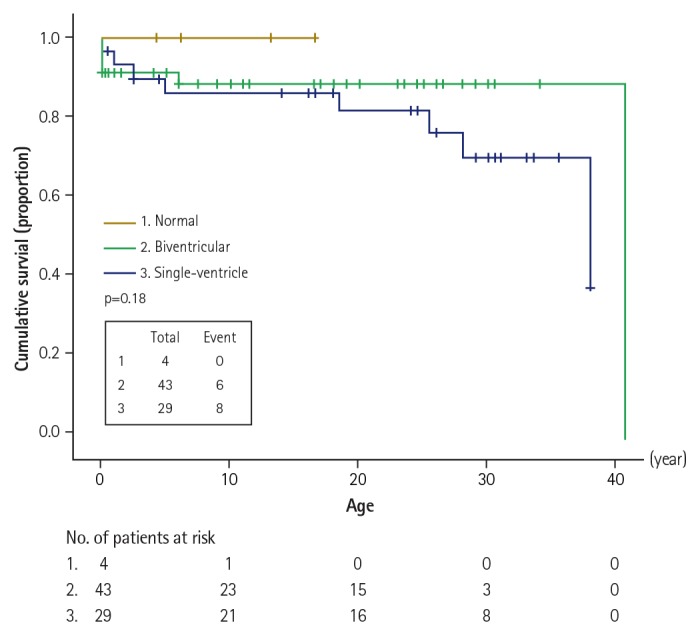
Table 1
Anatomical distributions of cardiovascular structures and primary diagnosis of cardiac anomalies in left isomerism
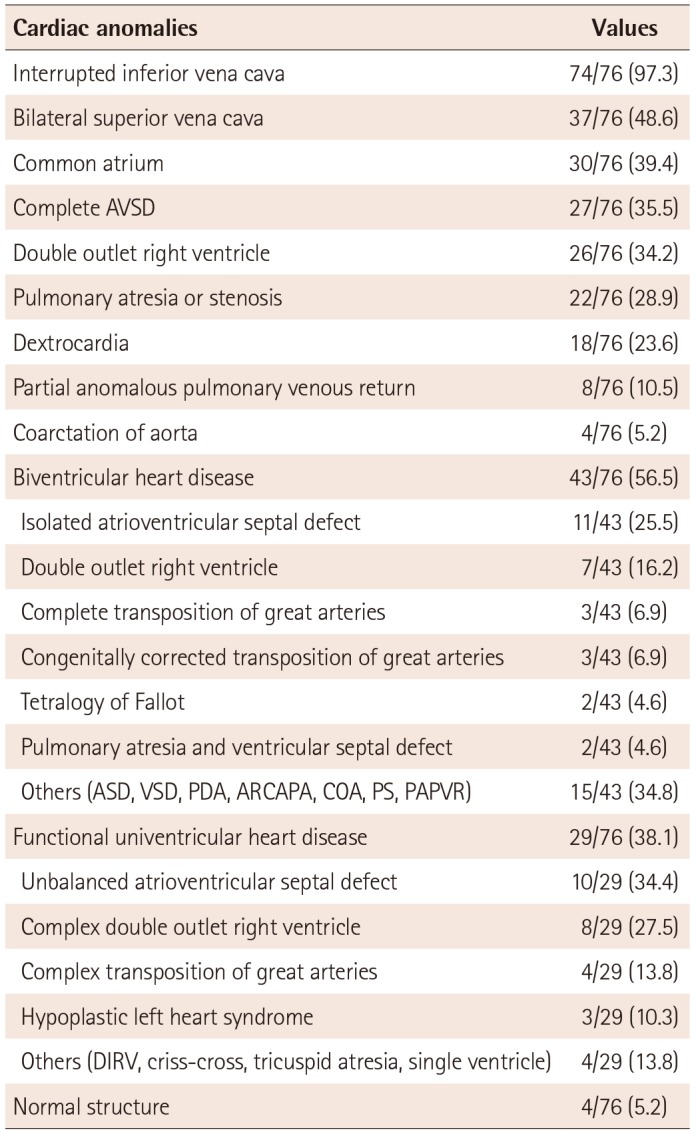
Values are presented as number (%). AVSD: atrioventricular septal defect, ASD: atrial septal defect, VSD: ventricular septal defect, PDA: patent ductus arteriosus, ARCAPA: anomalous right coronary artery arising from the pulmonary artery, COA: coarctation of aorta, PS: pulmonary stenosis, PAPVR: partial anomalous pulmonary venous return, DIRV: double inlet right ventricle
Table 2
Comparison data of interventions and late outcomes between biventricular repair and single-ventricle surgery groups
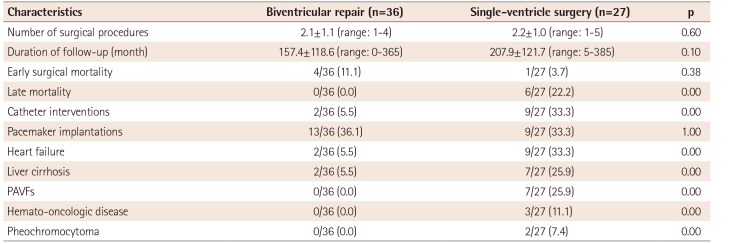
Table 3
Extracardiac disease and abnormalities
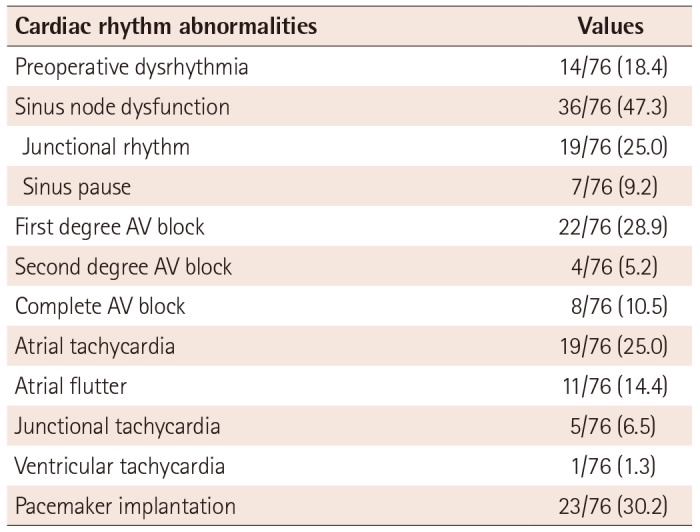
Table 4
Extracardiac disease and abnormalities
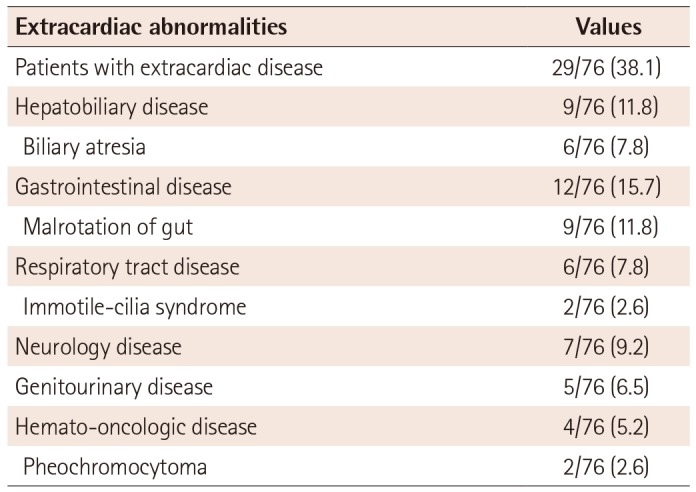
Table 5
Anatomy and outcomes in 7 patients with pulmonary arteriovenous fistulas
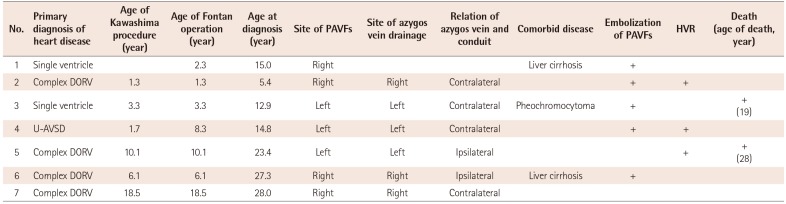
Table 6
Univariate analysis of risk factors for heart failure
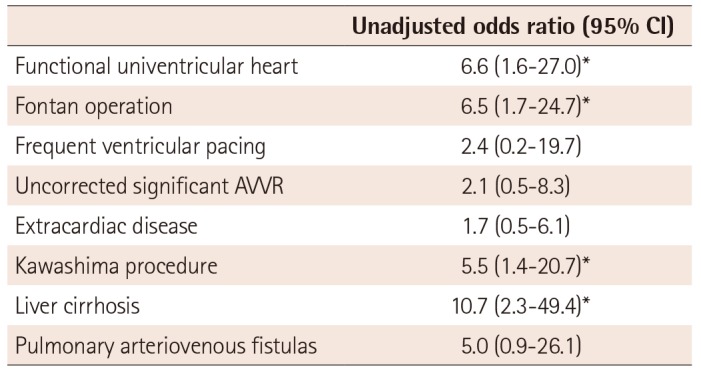
Table 7
Univariate estimates of risk factors for mortality




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download