Abstract
Background and Objectives
Behçet's disease (BD) is a systemic vasculitis that is characterized by genital, oral, or skin lesions, uveitis, and vascular complications. Studies have shown that increased arterial stiffness is common in systemic immune and inflammatory diseases such as rheumatoid arthritis and systemic lupus erythematosus. However, current research has not yet determined whether patients with BD have increased arterial stiffness. This meta-analysis compares arterial stiffness parameters in subjects with a BD diagnosis to normal subjects.
Subjects and Methods
A comprehensive search of the MEDLINE and EMBASE databases was performed from the database beginning through May 2016. Observation studies were included in this analysis if they assessed the association between BD and arterial stiffness in adult subjects. BD patients met the International Study Group criteria for a diagnosis of Behçet's disease. Aortic stiffness was assessed using carotid-femoral pulse wave velocity (PWV) measurements as an indicator. Pooled mean difference (MD) of PWV and 95% confidence intervals (CI) were calculated using a random-effect, generic inverse variance meta-analysis. The between-study heterogeneity of effect-size was quantified using the Q statistic and I2.
Results
Data were extracted from four observational studies that included 303 subjects. PWV is significantly higher in patients with Behçet's disease compared with controls (MD=0.74;95%, CI: 0.28-1.20, p=0.002, I2=63%).
Conclusion
In this meta-analysis, we observed that PWV, an ideal indicator of arterial stiffness, is increased in patients with Behçet's disease compared with the controls. Prospective studies in a large population should be done to determine the pathophysiological and prognostic implications of increased arterial stiffness in BD.
Behçet's disease (BD) is a systemic vasculitis of unknown etiology that is characterized by genital, oral, or skin lesions, uveitis, neurological, and vascular complications. It can cause venous thrombosis and arterial aneurysm. The frequency of vascular involvement is reported as 5-40%, depending on the literature source.1) However, current research has not yet determined the pathogenesis of BD. The histopathology results from studies involving arteries indicate that there is fibrous thickening in all layers of the vessel wall, with focal aneurysmal dilatation and thick thrombus formation over the aneurysms. Lymphocytic infiltration of the vaso vasorum, interrupting the medial elastic fibers has also been noted.1) Studies have shown that acute systemic inflammation and chronic systemic vasculitis are associated with endothelial dysfunction,2) which plays a major role in atherosclerosis in rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), contributing to the major cause of mortality.3) However the typical elements of vasculitis in BD patients are scarce or absent. It is thought that endothelial dysfunction in BD causes increased serum endothelium-derived vonWillebrand factor, soluble thrombomodulin, E selectin, and vascular endothelial growth factor.4)5)6)7) Subsequently, these endothelial findings lead to coagulation and fibrinolytic pathway abnormalities,7) nitric oxide (NO) activity impairment,8) increased vascular fibrosis and smooth muscle cell proliferation,9) and eventually lead to arterial stiffness as an end result. However, whether patients with BD have increased arterial stiffness is still debated in the literature. Thus, we conducted a systematic review and meta-analysis to compare arterial stiffness parameters in subjects with a BD diagnosis to normal subjects.
This systematic review and meta-analysis was conducted and reported according to the Meta-analysis of Observational Studies in Epidemiology Statement.10)
Two authors (Sanguankeo and Upala) independently searched published studies that were indexed in MEDLINE and EMBASE from database inception to May 2016. The following main search terms were used: Behçet syndrome, Behçet's syndrome, Behçet disease, vascular stiffness, arterial stiffness, aortic stiffness, pulse pressure, pulse wave velocity. The full search terms used are detailed in the Supplemental Data in the online-only Data Supplement. References of all selected studies were also examined.
This review included all published observational studies, including cross-sectional, prospective cohort, retrospective cohort and case-control studies that assessed the association of Behçet's syndrome and arterial stiffness. Reviews, case reports, and abstracts were excluded because the quality could not be assessed.
We included studies that recruited participants from the general population or used data from medical records from healthcare facilities. Participants were adults with Behçet's syndrome or healthy individuals that had measured arterial stiffness. We compared outcomes between patients who were diagnosed with Behçet's syndrome, based on the International Study Group criteria for diagnosis of Behçet's disease, and participants who did not have Behçet's syndrome. The main outcome of this study was aortic stiffness measured by pulse wave velocity (PWV).
Both authors independently reviewed titles and abstracts of all citations that were identified. After all abstracts were reviewed, data comparisons between the two investigators were conducted to ensure completeness and reliability. The inclusion criteria were independently applied to all identified studies. Differing decisions were resolved by consensus.
Full-text versions of potentially relevant papers identified in the initial screening were retrieved. Articles were assessed for data on the study design, source of information, participant characteristics, and Behçet disease and arterial stiffness assessment. We contacted the authors of the primary reports to request any unpublished data. If the authors did not reply, we used the available data for our analyses.
The Newcastle-Ottawa Scale (NOS) was used to evaluate subjective assessment of methodological quality for observational studies.11) The NOS is a quality assessment tool for non-randomized studies that uses a “star system” based on three major perspectives: study group selection (0–4 stars, or 0-5 stars for cross-sectional studies), group comparability, controlling for important and additional relevant factors (0–2 stars), and outcome of interest or exposure determination (0-3 stars). A total score of 3 or less was considered poor, 4-6 was considered moderate, and 7-10 was deemed high quality. We excluded studies from our meta-analysis if they were determined to be poor in quality. Discrepant opinions between authors were resolved by consensus.
We used the Comprehensive Meta-Analysis 3.3 software from Biostat, Inc. to perform a meta-analysis of the included studies. We calculated pooled mean difference (MD) of the PWV of aortic stiffness. All outcomes were reported as effect estimates, and associated 95% confidence intervals (CIs) were compared based on the results of Behçet disease, with normal groups using a random-effects model. We excluded studies from the meta-analysis and only presented the result with narrative description (qualitative analysis) when there was insufficient data for calculating pooled effect size. The heterogeneity of effect estimates across these studies was quantified using the Q statistic and I2 (p<0.10 was considered statistically significant). The Q statistic compared the observed between-study dispersion and expected dispersion of the effect size, and was expressed as a p value for statistical significance. I2 is the ratio of true heterogeneity to total observed variation. An I2 of 0% to 40% was considered to exclude heterogeneity, of 30% to 60% was considered to represent moderate heterogeneity, a value of 50% to 90% was considered to represent substantial heterogeneity, and 75% to 100% was considered to represent considerable heterogeneity.12) Publication bias was assessed using a funnel plot, Egger's regression test and the implications of the tests were assessed using the trim and fill method.13)
The initial search yielded 40 articles (Fig. 1); 29 articles were excluded because they were not original observational studies (12 articles), did not include data on Behçet disease (7 articles), or did not measure arterial stiffness using PWV as an outcome (10 articles). A total of 11 articles were included in the full-length review. Data were extracted from four studies that involved a total of 356 participants for qualitative analysis.14)15)16)17) The included studies varied in study location, sample size, and source of data. The characteristics of the four extracted studies included in this review are outlined in Table 1.
Four studies were included in the meta-analyses of carotid-femoral pulse wave velocity comparing Behçet disease and normal individuals (Fig. 2). There were no significant differences in the BMD between the two groups with a pooled MD of 0.74 (95% CI: 0.28-1.20, p=0.002, I2=63%).
To investigate potential publication bias, we examined the contour-enhanced funnel plot of the studies included in the meta-analysis of the lumbar spine (Fig. 3). The vertical axis represents study size (standard error) while the horizontal axis represents the difference in means. This plot demonstrates the presence of publication bias because there is an asymmetrical distribution of studies toward the right side of the mean. Additionally, the Egger's regression test did not indicate significant results (p=0.25). Based on the trim and fill methods for potential missing studies on the left side of the mean, we determined that there was no difference of the imputed MD and the associated 95% CI (MD=0.53; 95% CI: 0.15-1.92).
To the best of our knowledge, this is the first systematic review and meta-analysis that compares the arterial stiffness parameter in subjects diagnosed with BD and normal subjects. We observed that the PWV, an ideal indicator of arterial stiffness, increased in patients with BD, compared with controls. Currently, the etiologies of vascular disease in BD are not well understood. Vascular inflammation is the primary lesion in histopathology studies, and because of the endothelial cell injury, it appears to be the main precipitant of a prothrombotic state in BD. Multiple studies have demonstrated that endothelial cell injury with increased expression of proinflammatory and T-helper type 1 cytokines, adhesion molecules (VCAM-1 and ICAM-1), tissue factor, soluble CD40L, matrix metalloproteinase-9, and free oxygen radicals has led to abnormal coagulation and fibrinolytic activity; these studies also indicated that decreased NO release impairs flow-mediated dilatation and increased platelet reactivity and lipid peroxidation.7)8)18) The end result of this inflammatory cascade is vascular fibrosis and smooth muscle cell proliferation, which causes arterial stiffness and atherosclerosis. However, the data collected thus far have been conflicting.1)
Because very few studies have evaluated the risk of cardiovascular events in BD patients, the PWV was used as a surrogate test to investigate subclinical atherosclerosis. PWV is a reliable predictor of cardiovascular disease morbidity and mortality.19) PWV is defined as the velocity of the arterial pulse for movement along the vessel wall; it is also inversely related to the arterial distensibility or relative arterial compliance.20) It is calculated by measuring the time needed for pulse transit across the distance between two recording sites on the surface of the body, in meters, according to the following formula: PWV (m/s)=distance (m)/transit time (s).15) Alternatively, carotid intima media thickness (IMT) has also been used to evaluate arterial stiffness.21)22)
Caldas et al.,14) Chang et al.16) and Yilmaz et al.17) all found an increased PWV in patients with BD, however the results of a study by Kurum et al.15) did not support this finding. Caldas proposed that discrepancy in the findings was due to Kurum's patient group having a shorter disease duration and smaller sample size. However, we also found that the study by Kurum did not include patients with active BD. Kurum defined active BD as having signs of oral aphthae, genital ulcers, erythema nodosum, active neurologic involvement, and active gastrointestinal involvement. Therefore, we postulate that excluding active BD patients probably contributed to the non-significant findings in Kurum's study because these results were contrasted in a study by Yilmaz et al.,17) which reported that patients with active BD had higher PWV values than patients with inactive BD and the controls. PWV values were also higher in patients with active BD compared with patients with inactive BD.17)
Caldas et al.14) reported that carotid artery IMT was similar between BD and controls, but BD patients with systemic involvement had significantly higher PWV levels than those with only mucocutaneous involvement. There was also a higher PWV and IMT in BD patients with vascular involvement, but this result was not statistically significant.14) This finding was in agreement with some studies,8)23) but there are conflicting results.24)25)26) Some studies have found a higher IMT in BD patients, which is likely due to the presence of traditional risk factors, yet it could be due to impaired endothelial function before visible structural changes of the arterial wall in BD patients as proposed by Rhee et al.8)
In addition to PWV and IMT, the use of other non-invasive modalities has been evaluated. Balta et al.27) suggested that the mean platelet volume (MPV) could be a modality, because the MPV is a marker in other inflammatory diseases; he found that the MPV level was higher in BD patients that were positively correlated with CRP and PWV. In comparison, Celik et al.28) measured the arterial stiffness by calculating the augmentation index (AIx), which was positively correlated with the PWV. The AIx is calculated as the percent ratio of augmentation pressure (AG) to pulse pressure. AG is the accumulation of premature and increased pressure reflections superimposed over the pulse wave that is ejected from the heart.28) In addition, Uyar et al.29) found that carotid artery IMT, and coronary artery calcium scores were statistically higher and that the ankle-brachial pressure index (ABPI) was statistically lower in BD patients than in the control group. All of these parameters can also serve as non-invasive assessments of arterial stiffness.
There were some limitations to our study. First, PWV may not change significantly in the early atherosclerotic aorta because the aorta may still remain elastic.22) Second, the recording sites could affect the arterial stiffness accuracy estimate because carotid-radial PWV could underestimate the degree of arterial stiffness, because the radial artery is not subject to overt atherosclerosis to the same degree as the femoral artery.22) Despite the fact that the closest estimation of atherosclerosis severity can be obtained by measuring carotid femoral PWV, this value does not reflect the entire arterial tree measurement.22) Finally, all patients with BD were diagnosed based on the 1990 International Study Group (ISG) criteria, which is less sensitive and less accurate than the 2006 International Criteria for Behçet Disease (ICBD). The main difference between these two sets of criteria is that the ICBD criteria includes the presence of vascular manifestations (superficial phlebitis, deep vein thrombosis, large vein or arterial thrombosis, or aneurysm) and the presence of oral aphthosis is no longer required in the newer criteria. The ICBD criteria have been demonstrated to have better sensitivity (96.1% vs. 92%, p<0.001) and accuracy (93.8% vs. 92%) than the ISG criteria. Therefore, each study could have been able to increase their sample size if the new criteria had been used, in order to increase the power to detect the differences between the patient and control groups.30)
The implementation of these non-invasive modalities can improve the detection of arterial stiffness in patients with BD. However, studies included in this meta-analysis were mainly cross-sectional case-control studies. Prospective studies in a large population should be performed to determine the pathophysiological and prognostic implications of increased arterial stiffness in BD.
References
1. Seyahi E. Behçet's disease: how to diagnose and treat vascular involvement. Best Pract Res Clin Rheumatol. 2016; 30:279–295. PMID: 27886800.
2. Hingorani AD, Cross J, Kharbanda RK, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000; 102:994–999. PMID: 10961963.
3. Klocke R, Cockcroft JR, Taylor GJ, Hall IR, Blake DR. Arterial stiffness and central blood pressure, as determined by pulse wave analysis, in rheumatoid arthritis. Ann Rheum Dis. 2003; 62:414–418. PMID: 12695151.
4. Kayikçioğlu M, Aksu K, Hasdemir C, et al. Endothelial functions in behçet's disease. Rheumatol Int. 2006; 26:304–308. PMID: 15739096.
5. Haznedaroglu E, Karaaslan Y, Büyükaşik Y, et al. Selectin adhesion molecules in behçet's disease. Ann Rheum Dis. 2000; 59:61–63. PMID: 10627429.
6. Oztürk MA, Unverdi S, Oktar SO, et al. Vascular endothelial growth factor and carotid intima-media thickness in patients with behçet's disease. Clin Rheumatol. 2008; 27:961–966. PMID: 18204875.
7. Kiraz S, Ertenli I, Oztürk MA, Haznedaroğlu IC, Celik I, Calgüneri M. Pathological haemostasis and “prothrombotic state” in behçet's disease. Thromb Res. 2002; 105:125–133. PMID: 11958802.
8. Rhee MY, Chang HK, Kim SK. Intima-media thickness and arterial stiffness of carotid artery in Korean patients with behçet's disease. J Korean Med Sci. 2007; 22:387–392. PMID: 17596642.
9. Patel RS, Al Mheid I, Morris AA, et al. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011; 218:90–95. PMID: 21605864.
10. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000; 283:2008–2012. PMID: 10789670.
11. Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25:603–605. PMID: 20652370.
12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560. PMID: 12958120.
13. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001; 54:1046–1055. PMID: 11576817.
14. Caldas CA, Borba EF, Bortolotto LA, Medeiros DM, Bonfa E, Gonçalves CR. Increased arterial stiffness assessed by pulse wave velocity in behçet's disease and its association with the lipid profile. J Eur Acad Dermatol Venereol. 2013; 27:454–459. PMID: 22329367.
15. Kürüm T, Yildiz M, Soy M, Ozbay G, Alimgil L, Tüzün B. Arterial distensibility as determined by carotid-femoral pulse wave velocity in patients with behçet's disease. Clin Rheumatol. 2005; 24:134–138. PMID: 15365878.
16. Chang HK, Kim SK, Lee SS, Rhee MY. Arterial stiffness in behçet's disease: increased regional pulse wave velocity values. Ann Rheum Dis. 2006; 65:415–416. PMID: 16474038.
17. Yilmaz S, Celik G, Esmen SE. Assessment of arterial stiffness in patients with inactive and active behçet's disease. Scand J Rheumatol. 2014; 43:63–69. PMID: 24015673.
18. Butta NV, Fernández-Bello I, López-Longo FJ, Jiménez-Yuste V. Endothelial dysfunction and altered coagulation as mediators of thromboembolism in behçet disease. Semin Thromb Hemost. 2015; 41:621–628. PMID: 26276934.
19. Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the rotterdam study. Circulation. 2006; 113:657–663. PMID: 16461838.
20. Imura T, Yamamoto K, Kanamori K, Mikami T, Yasuda H. Non-invasive ultrasonic measurement of the elastic properties of the human abdominal aorta. Cardiovasc Res. 1986; 20:208–214. PMID: 3518941.
21. Izzo JL Jr. Arterial stiffness and the systolic hypertension syndrome. Curr Opin Cardiol. 2004; 19:341–352. PMID: 15218394.
22. Davies JI, Struthers AD. Pulse wave analysis and pulse wave velocity: a critical review of their strengths and weaknesses. J Hypertens. 2003; 21:463–472. PMID: 12640232.
23. Seyahi E, Ugurlu S, Cumali R, et al. Atherosclerosis in behçet's syndrome. Semin Arthritis Rheum. 2008; 38:1–12. PMID: 18221989.
24. Alan S, Ulgen MS, Akdeniz S, Alan B, Toprak N. Intima-media thickness and arterial distensibility in behçet's disease. Angiology. 2004; 55:413–419. PMID: 15258687.
25. Oztürk MA, Oktar SO, Unverdi S, et al. Morphologic evidence of subclinical atherosclerosis obtained by carotid ultrasonography in patients with behçet's disease. Rheumatol Int. 2006; 26:867–872. PMID: 16402216.
26. Hong SN, Park JC, Yoon NS, et al. Carotid artery intima-media thickness in behçet's disease patients without significant cardiovascular involvement. Korean J Intern Med. 2008; 23:87–93. PMID: 18646511.
27. Balta I, Balta S, Koryurek OM, et al. Mean platelet volume is associated with aortic arterial stiffness in patients with behçet's disease without significant cardiovascular involvement. J Eur Acad Dermatol Venereol. 2014; 28:1388–1393. PMID: 24164056.
28. Celik G, Yilmaz S, Ergulu Esmen S. Non-dipping blood pressure patterns and arterial stiffness parameters in patients with behçet's disease. Hypertens Res. 2015; 38:856–861. PMID: 26268564.
29. Uyar B, Solak A, Genç B, et al. Evaluation of arterial stiffness in patients with behçet's disease by using noninvasive radiological methods such as intima-media thickness of the carotid, ankle-brachial pressure index, coronary artery calcium scoring, and their relation to serum fetuin-a levels: a case-control study. Ann Dermatol. 2015; 27:702–708. PMID: 26719639.
30. Davatchi F. Diagnosis/classification criteria for behçet's disease. Patholog Res Int. 2012; 2012:607921. PMID: 21961081.
Supplementary Material
The online-only Data Supplement is available with article at https://doi.org/10.4070/kcj.2017.0004
Fig. 2
Forest plot of arterial stiffness (pulse wave velocity), for patients with and without Behçet's disease. CI: confidence interval.

Fig. 3
Funnel plots showing studies that reported arterial stiffness (pulse wave velocity) in patients with and without Behçet's disease. Circles represent observed published studies.
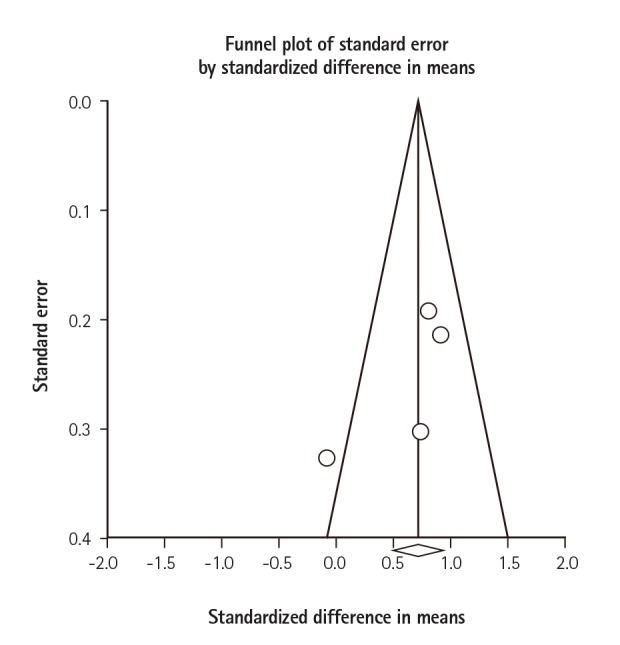
Table 1
Characteristics of the four extracted studies included in this review

| Study (year) | Design | Study duration | Source | Country | Participants (n) | Demographics | Diagnostic criteria | Outcome measurement methods | Result (PWV) | Study quality | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Women | Disease duration (year) | Patient | Control | |||||||||
| Caldas et al.14) | Cross-sectional case-control study | Not reported | Rheumatology division of the University of Sao Paulo | Sao Paulo, Brazil | 23 patients and 23 controls | 35.0±7.6 vs. 35.4±6.0 | 52% | 8.9±5.6 | International Study Group criteria | PWV, IMT | 8.4±1.1 | 7.5±1.4 | 8 |
| Chang et al.16) | Cross-sectional case-control study | Not reported | Not reported | South Korea | 53 patients and 65 controls | 38.1 vs. 38.2 | 49% | Not reported | International Study Group criteria | PWV | 7.9±1.1 | 7.2±0.6 | 7 |
| Kurum et al.15) | Cross-sectional case-control study | Not reported | Not reported | Erdine, Turkey | 14 patients and 28 controls | 32.1±7.4 vs. 27.9±6.1 | 28.57% | 6.64±4.5 | International Study Group criteria | PWV | 8.4±1.4 | 8.5±1.1 | 7 |
| Yilmaz et al.17) | Cross-sectional case-control study | Not reported | Rheumatology outpatient clinic | Konya, Turkey | 96 patients and 54 controls | 37.98±11.66 vs. 40.85±13.59 | 55.21% | 6.15±7.43 | International Study Group criteria | PWV, CO, MBP, SBP, DBP | 6.36±1.65 | 5.58±0.73 | 8 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download