Abstract
Spontaneous isolated superior mesenteric artery dissection (SISMAD) is an uncommon but potentially catastrophic pathology. Multiple classification schemes have been proposed for this occurrence. Although no consensus has emerged regarding which classification should be used, Li's classification scheme is more precise and complete compared to other classification systems and can be used to guide the treatment of SISMAD. Initial conservative treatment is promising, with favorable early and long-term outcomes for most patients; endovascular treatment is recommended for patients with persistent/recurrent symptoms after conservative treatment; surgical treatment should be performed without delay for patients with arterial rupture, intestinal necrosis, or failed endovascular treatment.
Spontaneous isolated superior mesenteric artery dissection (SISMAD) is defined as superior mesenteric artery (SMA) dissection without the presence of aortic dissection.1) Although this condition is considered rare, the development of advanced imaging technology, in particular computed tomography angiography (CTA), appears to have increased the detection of SISMAD in recent years.1)2)3)4)
Although significant advances in the understanding, diagnosis, and management of SISMAD have been made since the first reported case in 1947,5) no consensus has emerged regarding which classification and management strategy should be used.6) This study aims to review current SISMAD classification and management strategies to identify the optimal classification and management strategy for SISMAD.
SISMAD is a pleiomorphic disease, and a systematic approach requires adequate classification. The purpose of classification is to organize patients into groups according to one or more discriminating criteria. The resulting groups should be mutually exclusive and collectively exhaustive. Furthermore, the groups should be clinically informative in order to assist medical decision making. The main objective of classification schemes is to categorize patients into treatment groups rather than provide an exhaustive description of all possible types of SISMAD. Five classifications of SISMAD have been proposed over the recent years, including those proposed by Sakamoto et al,7) Yun et al,8) Zerbib et al,9) Luan et al,10) and Li et al.11)
The original classification proposed by Sakamoto is based on the imaging appearance of the false lumen. In this classification scheme, SISMAD was categorized into four types according to the appearance of the false lumen on imaging (Fig. 1).7) However, the method does not consider the true lumen's condition, which may be affected by thrombosis and stenosis. Thus, this classification does not account for SISMAD involving the total thrombotic occlusion of the SMA, although this subtype has been designated by Zerbib et al.9)
The classification proposed by Yun, based on angiographic findings, categorizes SISMAD into three types according to the presence of false luminal flow and true lumen patency at the dissected segment (Fig. 2).8) It is the simplest and most comprehensive scheme. However, according to Yun's report,8) a thrombosed false lumen with ulcer-like projection was not correlated with imaging type. Therefore, this classification method does not adequately incorporate cases involving thrombosed false lumens with ulcer-like projections.
The Zerbib classification is a modified version of Sakamoto's classification and is similarly based on the imaging appearance of the false lumen. Under this classification system, SISMAD is categorized into six types according to the presence of false luminal flow and true lumen patency at the dissected segment (Fig. 3).9) Compared with the other four classification systems, Zerbib's classification is the most complicated and complete. However, because it links the site of the primary intimal tear with the extent of the false lumen, this classification system does not describe the whole spectrum of SMA dissections. Indeed, SMA dissections with retrograde propagation of the false lumen to the SMA ostium are not addressed. Also, because type II often occurs with true lumen stenosis and thrombosed false lumen, types II and V should be one type.
The classification proposed by Luan, based on location and length of the dissection, categorizes SISMAD into four types (Fig. 4).10) It is useful for describing the location and length of the SMA dissection. However, it does not consider features of the true and false lumen such as shape, whether the false lumen is thrombosed, and the stenosis of the true lumen. Therefore, Luan and Li12) suggested that their classification scheme can well describe SISMAD when combined with Yun's classification scheme.8) For example, when a dissection is limited to the curved part of the SMA with a visible false lumen but without a visible re-entry site, it can be described as a type B-IIa, and when the dissection extends to the distal trunk of the SMA with thrombosed false lumen and occluded true lumen, it can be described as a type C-III.
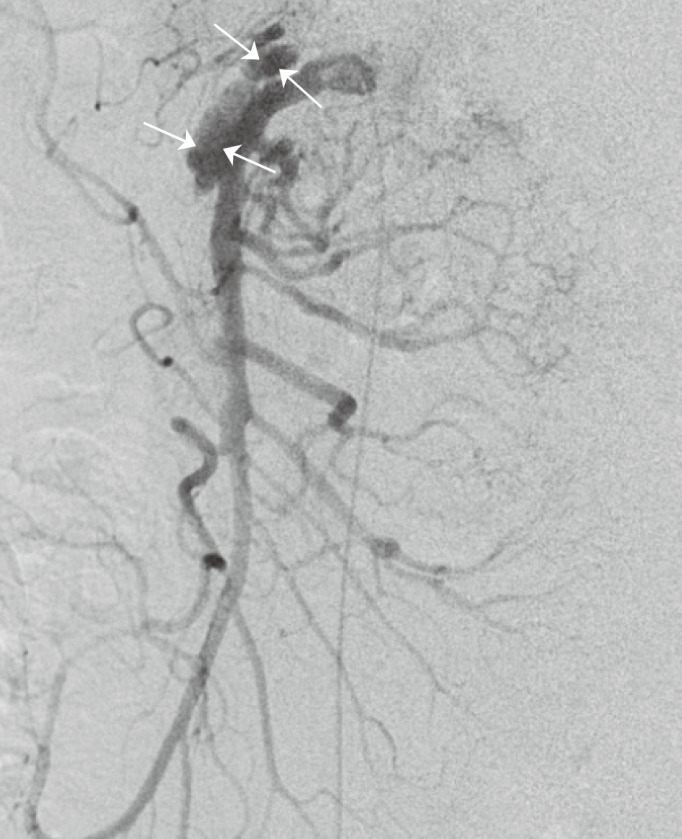
The Li classification system is based on the appearance on imaging of the true and false lumen and categorizes SISMAD into five types (Fig. 5).11) Similar to the other classification schemes, this method does not include all SISMAD subtypes, as SISMAD is a pleiomorphic disease. For example, one of our patients had two pseudoaneurysms of SISMAD (Fig. 6). However, the classification scheme by Li is more precise and complete than the other classification schemes.
The five classification schemes are all based on imaging appearance of the SISMAD: the view of radial point and whether the true lumen and false lumen are occluded or thrombosed. The main anatomical and physio-pathologic features of SISMADs are the location, the extent of the false lumen, and the distinction between thrombosed or not of the false and true lumen. All five classifications consider some of these anatomical features. However, these schemes either remain incomplete or appear impractical because of their complexity.
The main difference between the five classifications appears to be the distinction of the subtypes. Total thrombotic occlusion of the SMA is not included in Sakamoto's classification scheme,7) while Yun's classification scheme seems to be the simplest and most commonly used method.8) However, the major limitation of all of these systems that remains unaddressed is the incomplete anatomical description of the SISMAD.
Therefore, what is need is a simple categorization scheme that allows for exhaustive description of all anatomic types of SISMADs and that meets both the capabilities of modern imaging techniques and the demands resulting from an ever-growing treatment armamentarium. Although five classifications have been proposed to date, no consensus has been reached regarding which classification should be used. Currently, Li's classification scheme is more precise and complete compared to the others schemes and can be used to guide SISMAD treatment. Further work should be done to find an optimal classification of SISMAD.
The aim of SISMAD management is symptom relief and prevention of intestinal necrosis and rupture of the pseudoaneurysm. Although several treatment options, including conservative, endovascular, and surgical treatment, are available, there is currently no consensus regarding the optimal management of SISMAD.1)6)13141516)
Conservative treatment consists of bowel rest; nasogastric suction; intravenous fluid therapy; nutritional support; and antihypertensive, anticoagulation, and antiplatelet treatment.1)3) Antihypertensive treatment for SISMAD patients who are hypertensive is especially important. The rationale behind anticoagulation/antiplatelet treatment is to prevent thrombosis and possible distal embolization, especially in patients with very tight stenosis (>90%). However, anticoagulation/antiplatelet treatment does not impede progression of the dissection or aneurysmal enlargement in certain patients.17) Han et al.18) reported that long-term anticoagulation therapy can result in a high rate of complete remodeling during the natural course of symptomatic SIDSMA. However, Luan et al.3) reviewed the literature and found that the efficiency of conservative treatment with anticoagulation (77.2% [44 of 57]) is similar to that without anticoagulation (73.7% [112 of 152]; p>0.05). There is no evidence to identify the function of anticoagulation/antiplatelet in the treatment of SISMAD.
Initial conservative treatment is generally successful in patients with asymptomatic SISMAD, those with a patent dual-lumen artery with or without pseudoaneurysm formation, or those with SISMAD as long as the compromised lumen is adequate and/or adequate collaterals exist.1)3)11)18)19)20)21) In our previous study, we reported that conservative treatment can be applied successfully to most SISMAD patients.1) A recent systematic review, published before December 2014, which included all Chinese-language studies (including a total of 622 patients), reported that conservative treatment was successful in 63.2% of SISMAD patients.22) However, another systematic review of the English literature that included 143 published reports (including a total of 495 patients) reported that conservative treatment was successful in 86% of SISMAD patients.19) All studies, however, showed that conservative treatment can be applied successfully in most SISMAD patients.
The aims of endovascular treatment are to seal off the false lumen and re-establish the flow. This type of treatment was first reported by Leung et al.23) in 2000. The techniques include catheter-directed infusion of a vasodilator, balloon angioplasty, embolism ruptured branch of the SMA, embolism of the pseudoaneurysm, and stent placement.1)3)11)
The indications for endovascular treatment are disputed. Min et al.24) have suggested that indications include symptomatic patients with severe compression of the true lumen (>80%) and pseudoaneurysm >20 mm in diameter. Considering the disastrous consequences of intestinal necrosis, Luan et al.3) have suggested indications of persistence of abdominal pain or pseudoaneurysm >20 mm in diameter. In addition, patients who failed conservative treatment should undergo endovascular treatment.1)
The stenting technique is the most common in recent years, with a success rate of 97.6% (81/83).19) It is performed with endovascular techniques and does not change the circulation of the gut.25) The expected benefit of this approach is immediate exclusion of additional intimal tears in the SMA. This approach is appropriate for patients who fail conservative treatment, and recovery appears to be more rapid than in patients who undergo surgery. Stent placement can provide immediate symptom relief with shorter fasting time and good initial and middle-term results. However, little is known about the risk of restenosis or obstruction of the stented segment. Studies with longer follow-up periods are needed.
A self-expanding stent is recommended because of its good radial strength, flexibility, conformability, and sufficient length.1)3)26) Chu et al.27) suggested a covered stent for SISMAD with pseudoaneurysm. However, a covered stent can result in problematic coverage of some branches. A bare stent is sufficient for total exclusion of the dissecting pseudoaneurysm, sealing off the dissection and preserving the branches of the SMA.1)3)
Although coil embolization for dissecting pseudoaneurysms were reported,11) many SIDSMA patients with dissecting pseudoaneurysms have been treated by placement of self-expandable and open stents without coil embolization, with eventual uneventful discharge.1)19) We believe that a dissecting pseudoaneurysm would thrombose, and that pseudoaneurysm size would be reduced after placement of a stent, with gradual resolution of the false lumen and improved remodeling with patency of the true lumen. Also, there is a risk of rupture of the dissecting pseudoaneurysm with placement of coils, and the true lumen can be compressed if coils are used.28)
A recent systematic review that included 622 SISMAD patients reported that endovascular treatment was needed and successful in 33.6% of SISMAD patients.22) However, with greater understanding about SISMAD, increasing numbers of patients initially and successfully underwent conservative treatment, with endovascular treatment only performed in patients who failed conservative treatment.4)18)21) These data suggest that endovascular therapy should be reserved for cases in which conservative treatment has failed.
Surgical treatment was first described by Sisteron and Vieville in 1975.29) Since then, various surgical techniques have been reported, including aorto-mesenteric bypass, bypass between the SMA and right gastroepiploic artery, bypass between the SMA and right common iliac artery, interposition graft, intimectomy-patchplasty, transposition of the SMA to the aorta, intimectomy-thrombectomy, thrombectomy, endoaneurysmorrhaphy, ligation of dissecting pseudoaneurysm, and venous patchplasty.3)
Although many surgical techniques have been reported, conservative and endovascular treatments have become increasingly more popular than surgical treatments. This may be due to the following reasons: 1. surgical treatment is technically demanding; 2. surgical treatment is accompanied by significant trauma to the patient; 3. some patients who receive conservative or endovascular treatments cannot endure surgical treatment. Recently, a case of arterial rupture was successfully treated by stent placement.25) According to the literature, surgical treatment is proposed only for patients with arterial rupture, intestinal necrosis, or failed endovascular treatment.3)19) According to results from one systematic review, surgical treatment was needed only in 3.2% of SISMAD patients.22) That study suggested that surgical treatment should be performed in the small portion of patients with arterial rupture, intestinal necrosis, or failed endovascular treatment.
Although significant advances in the understanding, diagnosis, and management of SISMAD have been made, the incidence of mortality of SISMAD according to a systematic review was 1.6% (8/495), and all 8 patients in the study died before the diagnosis of SISMAD was made,19) which suggests that SISMAD is a potentially catastrophic pathology.
Recent options for conservative treatment and endovascular treatment have become popular, but it confusing as to when each option is applicable. Confusion arises from the fact that not all dissections are equal. Multiple algorithms for treatment have been published, but none include all aspects of pathology and treatment.2)6)19)20)21)22)23)24)25)26)27)28)29)30) Our suggested treatment algorithm for SISMAD is outlined in Fig. 7
Li's classification scheme is more precise and complete compared to the others and can be used to guide treatment of SISMAD. Initial conservative treatment is promising, with favorable early and long-term outcomes for most patients. Endovascular treatment is recommended for patients who have persistent/recurrent symptoms, and surgical treatment should be performed without delay for patients with arterial rupture, intestinal necrosis, or failed endovascular treatment.
Acknowledgments
This study was supported by the Natural Science Foundation of China (NO. 81401498), Jiangsu Provincial Medical Youth Talent (QNRC2016270) and by the High-Level Medical Talents Training Project (NO. 2016CZBJ009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
1. Jia ZZ, Zhao JW, Tian F, et al. Initial and middle-term results of treatment for symptomatic spontaneous isolated dissection of superior mesenteric artery. Eur J Vasc Endovasc Surg. 2013; 45:502–508. PMID: 23481411.
2. Kim HK, Jung HK, Cho J, Lee JM, Huh S. Clinical and radiologic course of symptomatic spontaneous isolated dissection of the superior mesenteric artery treated with conservative management. J Vasc Surg. 2014; 59:465–472. PMID: 24080130.
3. Luan JY, Li X, Li TR, Zhai GJ, Han JT. Vasodilator and endovascular therapy for isolated superior mesenteric artery dissection. J Vasc Surg. 2013; 57:1612–1620. PMID: 23538008.
4. Tomita K, Obara H, Sekimoto Y, et al. Evolution of computed tomographic characteristics of spontaneous isolated superior mesenteric artery dissection during conservative management. Circ J. 2016; 80:1452–1459. PMID: 27118619.
5. Bauersfeld SR. Dissecting aneurysm of the aorta; a presentation of 15 cases and a review of the recent literature. Ann Intern Med. 1947; 26:873–889. PMID: 20242656.
6. Ogino H. Current treatment strategy for spontaneous isolated dissection of the superior mesenteric artery. Circ J. 2016; 80:1323–1325. PMID: 27194374.
7. Sakamoto I, Ogawa Y, Sueyoshi E, Fukui K, Murakami T, Uetani M. Imaging appearances and management of isolated spontaneous dissection of the superior mesenteric artery. Eur J Radiol. 2007; 64:103–110. PMID: 17628380.
8. Yun WS, Kim YW, Park KB, et al. Clinical and angiographic follow-up of spontaneous isolated superior mesenteric artery dissection. Eur J Vasc Endovasc Surg. 2009; 37:572–577. PMID: 19208448.
9. Zerbib P, Perot C, Lambert M, Seblini M, Pruvot FR, Chambon JP. Management of isolated spontaneous dissection of superior mesenteric artery. Langenbecks Arch Surg. 2010; 395:437–443. PMID: 19588161.
10. Luan JY, Li X. Computed tomography imaging features and classification of isolated dissection of the superior mesenteric artery. Eur J Vasc Endovasc Surg. 2013; 46:232–235. PMID: 23746739.
11. Li DL, He YY, Alkalei AM, et al. Management strategy for spontaneous isolated dissection of the superior mesenteric artery based on morphologic classification. J Vasc Surg. 2013; 59:165–172. PMID: 23992995.
12. Luan JY, Li X. Response to ‘re. computed tomography imaging features and classification of isolated dissection of the superior mesenteric artery’. Eur J Vasc Endovasc Surg. 2014; 47:108–109.
13. Nomura Y, Yamaguchi M, Kitagawa A, Okada T, Okita Y, Sugimoto K. Hybrid management of ruptured isolated superior mesenteric artery dissecting aneurysm. J Vasc Surg. 2011; 54:1808–1811. PMID: 21741788.
14. Wagenhäuser MU, Sagban TA, Witte M, Duran M, Schelzig H, Oberhuber A. Isolated dissection of the superior mesenteric artery treated using open emergency surgery. World J Emerg Surg. 2014; 9:47. PMID: 25140196.
15. Park UJ, Kim HT, Cho WH, Kim YH, Miyata T. Clinical course and angiographic changes of spontaneous isolated superior mesenteric artery dissection after conservative treatment. Surg Today. 2014; 44:2092–2097. PMID: 24496981.
16. Li N, Lu QS, Zhou J, Bao JM, Zhao ZQ, Jing ZP. Endovascular stent placement for treatment of spontaneous isolated dissection of the superior mesenteric artery. Ann Vasc Surg. 2014; 28:445–451. PMID: 24070572.
17. Sparks SR, Vasquez JC, Bergan JJ, Owens EL. Failure of nonoperative management of isolated superior mesenteric artery dissection. Ann Vasc Surg. 2000; 14:105–109. PMID: 10742422.
18. Han Y, Cho YP, Ko GY, et al. Clinical outcomes of anticoagulation therapy in patients with symptomatic spontaneous isolated dissection of the superior mesenteric artery. Medicine (Baltimore). 2016; 95:e3480. PMID: 27100453.
19. Garrett HE Jr. Options for treatment of spontaneous mesenteric artery dissection. J Vasc Surg. 2014; 59:1433–1439.e1-2. PMID: 24655752.
20. Zhao Y, Yin H, Yao C, et al. Management of acute mesenteric ischemia: a critical review and treatment algorithm. Vasc Endovascular Surg. 2016; 50:183–192. PMID: 27036673.
21. Kim YW. Current understandings of spontaneous isolated superior mesenteric artery dissection. Vasc Specialist Int. 2016; 32:37–43. PMID: 27386450.
22. Luan JY, Guan X, Li X, et al. Isolated superior mesenteric artery dissection in china. J Vasc Surg. 2016; 63:530–536. PMID: 26597665.
23. Leung DA, Schneider E, Kubik-Huch R, Marincek B, Pfammatter T. Acute mesenteric ischemia caused by spontaneous isolated dissection of the superior mesenteric artery: treatment by percutaneous stent placement. Eur Radiol. 2000; 10:1916–1919. PMID: 11305570.
24. Min SI, Yoon KC, Min SK, et al. Current strategy for the treatment of symptomatic spontaneous isolated dissection of superior mesenteric artery. J Vasc Surg. 2011; 54:461–466. PMID: 21571493.
25. Lee JH, Ahn SG, Yoon J. Endovascular stent grafting via the left radial artery for a spontaneous isolated dissecting rupture of the superior mesenteric artery. Korean Circ J. 2012; 42:140–141. PMID: 22396706.
26. Yang HJ, Cho YK, Son TJ, Jung YY, Choi SA, Lee SH. Rapidly aggravated dissecting flap by angiography during percutaneous stent placement for acute isolated superior mesenteric artery dissection. Yonsei Med J. 2011; 52:859–862. PMID: 21786454.
27. Chu SY, Hsu MY, Chen CM, et al. Endovascular repair of spontaneous isolated dissection of the superior mesenteric artery. Clin Radiol. 2012; 67:32–37. PMID: 22070946.
28. Jia Z, Zhao J, Jiang G. Regarding “management strategy for spontaneous isolated dissection of the superior mesenteric artery based on morphologic classification”. J Vasc Surg. 2014; 59:876–877.
29. Sisteron A, Vieville C. Anevrysmes des arteres a destine digestive: Observations personnelles. In : Courbier R, editor. Chirurgie des arterio-pathies digestives. Paris: Expansion Scientifique Francaise;1975. p. 197–202.
30. Okamura K, Morizumi S, Kawata M, Suematsu Y. Conservative therapy as a primary treatment for spontaneous isolated dissection of the superior mesenteric artery. Ann Vasc Surg. 2014; 28:1939–1945. PMID: 25048807.
Fig. 1
Type I: patent false lumen with both entry and re-entry; Type II: ‘cul-de-sac’-shaped false lumen without re-entry; Type III: thrombosed false lumen with an ulcer-like projection, which is defined as a localized blood-filled pouch protruding from the true lumen into the thrombosed false lumen; Type IV: completely thrombosed false lumen with no ulcerlike projection.

Fig. 2
Type I: patent true and false lumens revealing entry and re-entry sites; Type II: ‘cul-de-sac’-shaped false lumen without re-entry; Type IIa: Visible false lumen but no visible re-entry site (blind pouch of false lumen); Type IIb: No visible false luminal flow (thrombosed false lumen); Type III: SMA dissection with occlusion of SMA. SMA: superior mesenteric artery.

Fig. 3
Type I: patent false lumen with both entry and re-entry; Type II: ‘cul-de-sac’-shaped false lumen without re-entry; Type III: thrombosed false lumen with an ulcer-like projection; Type IV: completely thrombosed false lumen with no ulcer-like projection; Type V: aneurismal dissection with stenosis of the distal part of the SMA; Type VI: total (VIa) or partial (VIb) thrombosis of the SMA. SMA: superior mesenteric artery.

Fig. 4
Type A: dissection localized to the curved part of the SMA and extended proximally to the SMA ostium; Type B: dissection limited to the curved part of the SMA; Type C: dissection localized to the curved part and extended distally, but the ileocolic artery or distal ileal artery was not involved; Type D: dissection localized to the curved part and extended distally to the ileocolic artery or distal ileal artery. SMA: superior mesenteric artery.

Fig. 5
Type I: patent false lumen with both entry and re-entry; Type II: ‘cul-de-sac’-shaped false lumen with no re-entry (subdivided into IIa, patent true lumen; IIb, severe stenosis of the true lumen; and IIc, occlusion of the true lumen); Type III: thrombosed false lumen with an ULP (subdivided into IIIa, patent true lumen; IIIb, severe stenosis of the true lumen; and IIIc, occlusion of the true lumen); Type IV: completely thrombosed false lumen with no ULP (subdivided into IVa, patent true lumen; IVb, severe stenosis of the true lumen; and IVc, occlusion of the true lumen); Type V: dissecting aneurysm. ULP: ulcer-like projection.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download