Abstract
Background and Objectives
Combination antiplatelet therapy reduces the risk of ischemic stroke compared with aspirin monotherapy in non-valvular atrial fibrillation (NVAF) patients. The underlying mechanism, however, remains unclear. In addition, the association between platelet inhibition and thrombogenicity in NVAF has not been evaluated.
Subjects and Methods
We randomized 60 patients with NVAF that were taking 100 mg of aspirin daily (>1 month) to adding 75 mg of clopidogrel daily (CLPD group), 100 mg of cilostazol twice daily (CILO group), or 1000 mg of omega-3 polyunsaturated fatty acid twice daily (PUFA group). Biomarkers (von Willebrand factor antigen [vWF:Ag], fibrinogen, D-dimer, and high-sensitivity C-reactive protein [hs-CRP]) and platelet reactivity (PR), which were the levels stimulated by adenosine diphosphate (ADP), thrombin-receptor agonist peptide, collagen, and arachidonic acid, were measured at baseline and 30-day follow-up.
Results
Combination antiplatelet therapy significantly reduced vWF:Ag and fibrinogen levels (7.7 IU/dL, p=0.015 and 15.7 mg/dL, p=0.005, respectively), but no changes were found in D-dimer and hs-CRP levels. The CLPD and CILO groups showed fibrinogen and vWF:Ag level reductions (24.9 mg/dL, p=0.015 and 9.3 IU/dL, p=0.044, respectively), whereas the PUFA group did not show any differences in biomarkers. Irrespective of regimen, the changes in fibrinogen and vWF:Ag levels were mainly associated with the change in ADP-mediated PR (r=0.339, p=0.008 and r=0.322, p=0.012, respectively).
Atrial fibrillation (AF) is the most common sustainable arrhythmia that significantly increases the risk of systemic thromboembolism, including stroke.1) Multiple factors, such as stasis, endocardial dysfunction/damage, inflammation, platelet dysfunction and abnormal coagulation factors, contribute to a pro-thrombotic milieu in AF patients, which frequently produces systemic embolic events in this patient population.1) Although activation of the coagulation cascade is a major contributor to thrombus formation in AF, platelet activation initiates the coagulation cascade by activating tissue factor (extrinsic pathway) and factor XII (intrinsic pathway).2)3) Moreover, most thrombin generation occurs after initial thrombus formation by activated platelets.3) Based on these findings, aspirin has long been used for stroke prevention in patients with non-valvular AF (NVAF).4)5) However, aspirin has a weak antiplatelet agent that represents only a 20% reduction in stroke.4)5)
Clinical use of novel oral anticoagulants (NOACs) in NVAF patients is rapidly increasing due to a better safety profile and a similar clinical efficacy, compared with an oral vitamin K antagonist (VKA).4)5) However, NOACs still have clinical issues in regards to safety, cost, and lack of antidotes. NOACs were associated with a higher risk of gastrointestinal bleeding.6) In addition, approval of factor Xa inhibitor antidote by the US Food and Drug Administration is still pending. NOACs are limited for treating patients with chronic kidney disease, in whom studies showed both high bleeding diathesis and thrombotic tendencies.4)5) Indeed, NOACs are not recommended for patients with severe renal impairment and dose adjustments are mandatory for those with moderate renal impairment.4)5) The current guidelines do not provide adequate recommendations for resumption after a serious bleeding event.4)5) These drawbacks could reduce wide acceptance of NOACs in real clinical practice. For example, in a large scale observation study, 36.9% of patients with CHA2DS2-VASc score ≥2 were not treated with anticoagulants but with antiplatelet agents.7)
AF is less common in Asian populations than in the rest of the world.8) However, Asian AF patients have a higher risk of stroke and a greater mortality than Caucacian.9) The types of stroke experienced by Asian patients appear to be different from those in Caucasians, which represent a greater proportion of hemorrhagic strokes among overall events.9) In addition, Asian patients have shown a higher prevalence for intracerebral bleeding than non-Asians, even with a low target international normalized ratio, which in turn results in some resistance to warfarin use in Asian populations.9)10)11) These findings lead to the over-prescription of aspirin monotherapy in Asian countries for primary and secondary prevention of stroke.11) However, the extent of platelet contribution to thrombus formation in AF remains unclear.12)13) Recent clinical data have demonstrated that combined antiplatelet therapy can reduce the risk of clinical events in NVAF patients compared with aspirin monotherapy.14) Interestingly, combination antiplatelet therapies reduced the risk of vascular death (hazard ratio [HR]=0.79; 95% confidence interval [CI]=0.64 to 0.97) in Korean patients that suffered from cardioembolic stroke (n=6197), without increasing the risk of hemorrhagic stroke-related death (HR=0.97; 95% CI=0.38 to 2.45), compared with antiplatelet monotherapy.15) Because intensified inhibition of platelet activation with combination antiplatelet therapy versus aspirin monotherapy may not be sufficient to explain the latter findings, other underlying mechanisms are required to explain this observation.
Some drugs with antiplatelet properties (e.g. clopidogrel, cilostazol, and omega-3 polyunsaturated fatty acids [PUFA]) have shown preventive effects against stroke-related clinical events.14)15)16)17) Although adding these agents to aspirin increases the level of platelet inhibition compared with aspirin monotherapy, the effects of combination antiplatelet therapies on the prothrombotic milieu in NVAF have not been studied in an Asian population.18)19)20) Therefore, this study was performed to evaluate whether intensive platelet inhibition, using combination antiplatelet therapies with clopidogrel, cilostazol, and PUFA, could modulate thrombogenicity in NVAF patients, compared to aspirin monotherapy.
The present study was a single-center, prospective, randomized, open label parallel-group study. We prospectively enrolled patients who had permanent NVAF, from July 2010 to March 2013. Permanent NVAF was confirmed based on electrocardiographic findings on at least 2 separate occasions (≥4 weeks apart). The patients were eligible if they had a CHA2DS2-VASc score ≤1, or were unsuitable for or unwilling to take oral anticoagulants. All patients were treated with 100 mg of aspirin daily for at least 1 month before enrollment. Major exclusion criteria were: age <20 years, valvular AF, contraindication for clopidogrel, cilostazol, or PUFA, active bleeding and bleeding diatheses, left ventricular ejection fraction <30%, leukocyte count <3000 cells/µL, platelet count <100000 cells/µL, aspartate aminotransferase or alanine aminotransferase level ≥3 times upper normal values, previous intracerebral hemorrhage, non-cardiac disease with a life expectancy <1 year, and inability to follow the protocol.
Baseline blood samples were collected during chronic aspirin administration (Fig. 1). The patients were then randomly allocated to the addition of 75 mg of clopidogrel daily (Plavix®, Sanofi-Aventis; CLPD group), 100 mg of cilostazol twice daily (Pletaal®, Otsuka Korea), or 1000 mg of PUFA twice daily (Omacor®, Kunil Pharmacy, Seoul, Korea) for 30 days in addition to aspirin therapy. No changes in other medications were allowed during the study period. At 30-day follow-up, drug adherence and adverse events were assessed by the attending physician, based on interview, pill counting, a questionnaire, and laboratory evaluation. If patients showed complete compliance, follow-up blood samples were obtained. The Institutional Ethics Committee approved the study protocol, and of the all study patients provided written informed consent.
Blood samples were drawn from an antecubital vein and collected in plastic tubes that contained 3.2% sodium citrate (Becton-Dickinson, San Jose, CA, USA) for plasma and a serum separator tube (Becton-Dickinson) for serum.
We tested four different types of well-known thrombogenic biomarkers that are related to adverse cardiovascular events in NVAF patients.1) These included von Willebrand factor antigen (vWF:Ag) as a marker of endothelial or endocardial dysfunction/damage in plasma, D-dimer and fibrinogen as markers of coagulation activity, and high sensitivity C-reactive protein (hs-CRP) as a marker of inflammation in serum. Plasma and serum blood samples for plasma marker quantification were separated within 1 hour of collection by centrifugation at 1500 to 2000×g for 15 minutes. Aliquots were stored at −70℃ to allow batch analysis. The vWF:Ag was measured by enzyme-linked immunosorbent assay (ELISA) with the REAADS von Willebrand factor antigen kit (Corgenix, Broomfield, CO, USA) and a Sunrise™ microplate reader (Tecan, Grödig, Austria). Fibrinogen was measured by the Clauss method with STA-Fibrinogen reagent (Diagnostica Stago, Paris, France), and D-dimer levels were measured by the immunoturbidimetric method with the STA-Liatest D-DI reagent (Diagnostica Stago), using a Stago STA-R Evolution® hemostasis system (Diagnostica Stago). The hs-CRP level was measured with the immunoturbidimetric method, using an automated chemistry analyzer (Modular DP; Roche Diagnostics, Mannheim, Germany). Intra- and inter-assay coefficients of variation for ELISA were <5% and <10%, respectively. The absolute changes in each thrombogenic biomarker were calculated as follows: Δthrombogenic biomarker=the level of thrombogenic biomarker on aspirin monotherapy-the level of thrombogenic biomarker on 30-day combination antiplatelet therapy.
Light transmittance aggregometry was performed within 2 hours of blood sampling, according to a standard protocol. The validation data have been described elsewhere.21) Plateletrich plasma (PRP) was obtained after centrifuging blood samples at 120×g for 10 minutes. The remaining blood was further centrifuged at 1200×g for 10 minutes to recover platelet-poor plasma (PPP). PRP was adjusted to a platelet count of 250000 cells/mm3 by adding PPP, if needed. Platelet reactivity (PR) was evaluated for 10 minutes at 37℃ using an AggRAM aggregometer (Helena Laboratories Corp., Beaumont, TX, USA), by laboratory personnel blinded to the study protocol. PR was determined as the maximal extent of platelet aggregation after adding 10 µM ADP, 25 µM thrombin receptor agonist peptide (TRAP), 6 µg/mL collagen, and 0.5 mg/mL arachidonic acid (AA). The absolute change in PR (ΔPR) was calculated as follows: ΔPR=PR on aspirin monotherapy-PR on 30-day combination antiplatelet therapy.
The primary endpoint was changes in the level of vWF:Ag during aspirin monotherapy and after each combination antiplatelet therapy.1) Secondary endpoints were: (1) changes in the fibrinogen, D-dimer, and hs-CRP levels; (2) PR value changes (stimulated by ADP, TRAP, collagen, and AA); (3) the correlation between the levels of thrombogenic markers and PRs during aspirin monotherapy; and (4) the correlation between changes in thrombogenic markers and in PR after each combination therapy.
Assuming a 25% relative difference in vWF:Ag level between aspirin monotherapy and each combination therapy, at least 17 patients were needed to demonstrate 80% power and a 2-tailed significance level of 0.05 with a 35% standard deviation (PS program version 3.0.14). Expecting a 20% drop out of enrolled patients, we determined that 20 patients were needed for each group.
Continuous variables were expressed as mean±standard deviation, and categorical variables were expressed as percentages. The Student's unpaired t or Mann–Whitney U test, and ANOVA or Kruskal Wallis test were used to compare continuous variables, and comparisons between categorical variables were performed using Chi-square statistics or Fisher's exact test, as appropriate. Comparisons of platelet function and thrombogenic markers between baseline and follow-up were performed with the Student's paired t-test or Wilcoxon signed rank test, as appropriate. The pharmacodynamic effect of different regimens was compared with a linear mixed model, taking into account the within-subject correlation and a first-order autoregressive error structure. Each model included treatment, time, an interaction between treatment and time, and the baseline pharmacodynamic parameter as a covariate. Least squares (LS) estimates of the mean difference, which assess parameters by minimizing the squared discrepancies between observed data and their expected values, are presented with 95% CIs and a two-tailed p value for the treatment effect. A multivariate linear regression model was employed to test for an independent linkage between clinical, echocardiographic, or platelet function measures and the changes in thrombogenic biomarkers. Independent variables were selected from the CHA2DS2-VASc scores and known variables with reported significant influence on the thrombogenicity of NVAF.1)4) A p value<0.05 was considered statistically significant, and statistical analyses were performed using SPSS ver. 21.0 software (SPSS Inc., Chicago, IL, USA).
A total of 65 NVAF patients were enrolled and 5 patients were excluded from the initial analysis due to incomplete compliance (n=3) and withdrawal due to headache (n=2) (Fig. 1). For the total cohort, the mean age was 63.5±9.0 years and about two-thirds of the participants were male (Table 1). Approximately half of the patients (n=26, 43.3%) showed a CHA2DS2-VASc score ≥2. Among them, 7 patients had a prior history of serious bleeding events related to a VKA use, and 12 patients were not suitable for VKA use due to the refusal of regular blood monitoring. In addition, 7 patients with poor control of the international normalized ratio, which was mainly associated with poor drug adherence, were also enrolled in the study. Baseline clinical and laboratory characteristics were well matched between the groups.
During aspirin monotherapy, the levels of vWF:Ag, fibrinogen, D-dimer, and hs-CRP were 141.1±33.9 IU/dL, 288.0±57.9 mg/dL, 509.5±722.5 ng/mL, and 112.0±102.7 µg/dL, respectively (Table 2). In addition, the mean values of PRs mediated by ADP, TRAP, collagen, and AA were 67.5±12.9%, 62.6±16.5%, 63.7±15.6%, and 24.1±17.8%, respectively (Table 3). Patients with high CHA2DS2-VASc scores (>2) overall showed higher levels of thrombogenic biomarkers and PRs compared with patients with low scores (≤2) (Fig. 2). The levels of ADP-mediated PR and coagulation markers (fibrinogen and D-dimer) were significantly higher in patients with high CHA2DS2-VASc scores (>2) than in those with low scores (≤2) (p=0.014 for ADP-induced PR, p=0.014 for fibrinogen, and p<0.001 for D-dimer, respectively) (Fig. 2).
The vWF:Ag and fibrinogen levels were significantly reduced after 30-day combination antiplatelet therapy (7.7 IU/dL; p=0.015 and 15.7 mg/dL; p=0.005, respectively), but no changes were found in D-dimer or hs-CRP levels (Fig. 3). The changes in thrombogenic markers also differed, depending on the regimens used (Table 2). Compared with aspirin monotherapy, adding clopidogrel or cilostazol generally reduced the levels of vWF:Ag (12.1 IU/dL; p=0.081 for clopidogrel and 9.3 IU/dL; p=0.044 for cilostazol, respectively) and fibrinogen (24.9 mg/dL; p=0.015 for clopidogrel and 11.4 mg/dL; p=0.151 for cilostazol, respectively). However, adjunctive PUFA did not decrease the levels of any thrombogenic biomarkers (Table 2).
In a subgroup of CHA2DS2-VASc scores ≥2, combination antiplatelet regimens did not significantly decrease thrombogenic markers, while combination therapy with clopidogrel showed numerical reduction of the fibrinogen level (28.2 mg/dL; p=0.071) (Supplementary Table 1 in the online-only Data Supplement).
The changes in PR levels differed according to the medication regimens after 30-day combination antiplatelet therapy (Table 3). Compared with aspirin monotherapy, adding clopidogrel mainly reduced the level of ADP-mediated PR (LS mean difference=16.1%; 95% CI of difference=6.0% to 26.4%; p=0.004), whereas adding PUFA significantly decreased the level of collagen-mediated PR (LS mean difference=8.4%; 95% CI of difference=0.8% to 16.1%; p=0.033). Of note, adjunctive cilostazol in addition to aspirin significantly reduced the PR levels stimulated by all agonists.
During aspirin monotherapy, the levels of fibrinogen were significantly correlated with only ADP-mediated PRs (r=0.349, p=0.006), while hs-CRP values were linked to all PR values (r≥0.262, p≤0.043) except TRAP-mediated PR (r=0.127, p=0.976) (Table 4). However, there were no significant relationships between vWF:Ag/D-dimer and PR levels.
After 30-day combination antiplatelet therapy, the changes in ADP-mediated PR were significantly associated with the changes in vWF:Ag (r=0.322, p=0.012) and fibrinogen (r=0.339, p=0.008) (Fig. 4). In a subgroup analysis that included patients with a CHA2DS2-VASc score ≥2, only fibrinogen level changes were correlated with changes in ADP-mediated PR (r=0.396, p=0.045) (Supplementary Table 2 in the onlineonly Data Supplement). In addition, changes in collagen-mediated PR were significantly correlated with changes in the D-dimer level (r=0.404, p=0.041) (Supplementary Table 2 in the online-only Data Supplement). However, the changes in PRs mediated by other agonists did not influence changes in any thrombogenic biomarkers (data not shown). Multivariate analysis showed the change in ADP-mediated PR by combination antiplatelet therapy was the only determinant of a decrease in vWF:Ag (β coefficient=0.396; standard error [SE]=0.188; p=0.018) and fibrinogen (β coefficient=0.818; SE=0.349; p=0.024) over time, when adjusted for CHA2DS2-VASc scores, age, white blood cell count, platelet count, hematocrit, glomerular filtration rate, low-density lipoprotein cholesterol, left ventricular ejection fraction, and left atrial volume index.
This was the first study to evaluate the effect of combination antiplatelet therapy on thrombogenic marker levels and platelet reactivity in East Asians with NVAF. The key findings of the study are as follows: (1) a high CHA2DS2-VASc score (>2) was associated with higher levels of D-dimer, fibrinogen, and ADP-mediated PR, compared with a low-score (≤2); (2) although any combination antiplatelet regimens did not reduce the levels of D-dimer and hs-CRP, adding clopidogrel or cilostazol significantly reduced the levels of vWF:Ag and fibrinogen; (3) clopidogrel, an ADP P2Y12 receptor inhibitor, reduced the level of ADP-mediated PR, whereas cilostazol significantly decreased the PR levels stimulated by multiple agonists; and (4) during combination therapy, the changes in fibrinogen and vWF:Ag levels were significantly correlated with the change in ADP-mediated PR, which may indicate a close linkage between ADP-mediated PR and prothrombotic milieu in NVAF patients.
The benefit of adding clopidogrel to low-dose aspirin in NVAF patients was well documented in the placebo-controlled ACTIVE-A (Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events-A) trial and the large-scale Korean Stroke Registry (n=7554).14)15) However, combination therapy was definitely inferior to warfarin for stroke prevention in NVAF patients.22) The data from the Korean Stroke Registry also showed that combination antiplatelet therapy was not superior to warfarin for secondary prevention after ischemic stroke that was related to AF (HR=0.79; 95% CI=0.64 to 0.97 for combination antiplatelet therapy vs. HR=0.66; 95% CI=0.59 to 0.74 for warfarin, both compared with aspirin monotherapy).15) The present study suggests the possible mechanism for the limited efficacy of combination antiplatelet therapy for stroke prevention in NVAF patients. Combination antiplatelet therapy was not effective in reducing D-dimer levels of NVAF patients, whereas, D-dimer levels were effectively reduced by oral anticoagulants.23) Indeed, D-dimer is considered the gold standard biomarker out of biomarkers used to predict stroke risks in NVAF patients.1)24) In the meta-analysis by Wu et al.,24) the high D-dimer levels were associated with 3-fold increased risk of subsequent thromboembolic events in NVAF patients. In addition, D-dimer levels increased with the accumulation of clinical risk factors for thromboembolism or with the presence of atrial thrombi.1) The results of this study also demonstrated that the patients with high CHA2DS2-VASc scores (>2) showed higher D-dimer levels than those with low scores (≤2). These findings suggest that combination antiplatelet therapy may be unsuitable for NVAF patients at high thromboembolic risk.
International guidelines for antithrombotic regimens are entirely dependent on the CHA2DS2-VASc scoring system.4)5) According to this system, antithrombotic therapy is not preferred for patients with a CHA2DS2-VASc score=0, while only aspirin is recommended as an antiplatelet therapy option for AF patients with a CHA2DS2-VASc score=1.4)5) However, the predictive power of the CHA2DS2-VASc score is not as strong as could be expected (C statistic=0.606, 95% CI=0.513–0.699), and not all patients with low CHA2DS2-VASc scores were as safe as the score might indicate.4)5)25) The 1-year thromboembolic event rate of patients with a CHA2DS2-VASc score=1 range from 0% (comparable to CHA2DS2-VASc score=0) to 3.4% (comparable to CHA2DS2-VASc score=3).4)5)25) In addition, about one-third of NVAF patients were classified as low-risk patients, i.e., with a CHA2DS2-VASc score=0 or 1.4)5)25) This suggests that a substantial number of NVAF patients might benefit from antithrombotic therapies. Given that the thrombogenic biomarkers tested in this study have been closely associated with thromboembolic events in AF patients, combination antiplatelet therapy with clopidogrel or cilostazol might be an option for managing patients with CHA2DS2-VASc scores=1 and high levels of vWF:Ag or fibrinogen.
Age >65 years is one of the most important determinants of both stroke and bleeding risks.4)5) Elderly patients aged 65–74 years had the largest benefit when clopidogrel was added to aspirin monotherapy in the ACTIVE-A study.14) This suggests that patients who are 65-74 years of age and have a high HAS-BLED score might be the best candidates for combination antiplatelet therapy in Asian NVAF patients. In addition, clopidogrel plus aspirin showed better cost-effectiveness than aspirin monotherapy in patients with a CHADS2 score ≥2 and a major bleeding risk <2.5%/patient-year, which corresponds to a HAS-BLED score of 2 to 3.4)5)
This study showed that ADP-mediated PR was closely linked to the levels of vWF:Ag and fibrinogen, which are important predictors of thromboembolic and atherothrombotic events.1) This finding suggests that a P2Y12 inhibitor may be a key antiplatelet regimen for patients that require a combination antithrombotic regimen with an anticoagulant and antiplatelet agents. Importantly, 5–8% of patients undergoing PCI need triple antithrombotic therapy (TAT) consisting of an oral anticoagulant plus dual antiplatelet therapy to prevent both thromboembolic events related to AF, and ischemic recurrences of coronary artery disease.4) However, TAT is associated with a significant increase of bleeding complication.4) Therefore, the balance between the risk of thromboembolic/atherothrombotic events and bleeding is very important for these patients and should be carefully considered. Recently, the WOEST (What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing) study revealed new information about the increased risk of bleeding that is associated with TAT.26) The study demonstrated that dual antithrombotic therapy with an oral anticoagulant and clopidogrel was associated with less bleeding and comparable ischemic events, compared with conventional TAT.26) Based on these results, current European guidelines recommend dual antithrombotic therapy with an oral anticoagulant plus a single antiplatelet agent (preferably clopidogrel) after 1–6 month TAT in patients with AF that are undergoing coronary stenting.4) The guideline also recommends single antithrombotic therapy with an oral anticoagulant after 12 months.4) However, concerns remain about the long-term efficacy of single antithrombotic therapy in high risk patients. Indeed, a residual risk of ischemic recurrences, including cardiovascular mortality, persists even beyond 1 year after myocardial infarction.27) Given the relative safety of dual antithrombotic therapy shown in the WOEST study, adding clopidogrel to an oral anticoagulant may be a better option for NVAF patients with myocardial infarction than a single oral anticoagulant. Moreover, the present study suggests that clopidogrel could provide additive benefits to these patients, and improve endothelial dysfunction.
The present study evaluated changes in biomarkers. Therefore, we could not prove the clinical efficacy of combination antiplatelet therapy. However, given that biomarkers in NVAF are well-known predictors of systemic embolization, the study findings could provide important background for selecting combination antiplatelet therapy in patients that are not suitable for oral anticoagulants. NOACs might be more efficacious than DAPT, even in patients with low CHA2DS2-VASc scores. However, concerns about Asian heterogeneity still persist even after the development of NOACs.9) Indeed, clinical evidence has suggested that Asians have a higher risk of serious bleeding during NOAC treatment than non-Asians. A therapy of 150 mg of dabigatran twice daily showed almost a 2-fold risk of hemorrhagic stroke in Asians, compared with non-Asians (0.17%/year vs. 0.09%/year).28) When compared with the results of the global study that used 20 mg of rivaroxaban daily, Japanese patients that received a lower dose of ribaroxaban (15 mg daily) showed a higher incidence of clinically relevant bleeding (14.9%/year in the global study cohort vs. 18.0%/year in the Japanese cohort).29)30) Therefore, antiplatelet therapy continues to have a role in some clinical conditions, as previously discussed. The present study did not test the efficacy of potent P2Y12 inhibitors, such as prasugrel and ticagrelor. However, the correlation between ADP-induced PR inhibition and reduction of fibrinogen and vWF:Ag suggests the potential role of potent P2Y12 inhibitors in some NVAF patients. Future studies that evaluate NOACs in patient with AF undergoing PCI will answer the possible roles of combined NOACs and potent P2Y12 inhibitors therapy.
Combination antiplatelet therapy was partially effective in reducing a pro-thrombotic milieu in Korean NVAF patients because it reduced the vWF:Ag and fibrinogen levels but not D-dimer and hs-CRP levels. Because changes in vWF:Ag and fibrinogen levels were determined primarily by the inhibitory levels of ADP-induced PR rather than the combination antiplatelet regimen, it is possible that P2Y12 inhibitors might be key antiplatelet agents in NVAF patients. However, clopidogrel did not sufficiently reduce the vWF:Ag level, possibly due to weak inhibition of the P2Y12 receptor. This warrants future study to evaluate the efficacy of potent P2Y12 inhibitors in NVAF patients. The clinical implications of these findings need to be further evaluated in future trials.
Figures and Tables
Fig. 1
Study flow diagram. AF: atrial fibrillation, CLPD: clopidogrel, CILO: cilostazol, PUFA: polyunsaturated fatty acid.

Fig. 2
Differences in platelet reactivity (A) and thrombogenic biomarkers (B) during aspirin monotherapy, according to the CHA2DS2-VASc scores. Red and grey bars represent the values in patients with low and high CHA2D2-VASc scores, respectively. The error bars denote the standard deviation. ADP: adenosine diphosphate, TRAP: thrombin receptor agonist peptide, Coll: collagen, AA: arachidonic acid, vWF:Ag: von Willebrand factor antigen, Fib: fibrinogen, hs-CRP: high sensitivity-C reactive protein.
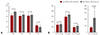
Fig. 3
Thrombogenic biomarker levels during aspirin monotherapy and combination antiplatelet therapy. vWF:Ag: von Willebrand factor antigen, hs-CRP: high sensitivity-C reactive protein.

Fig. 4
Changes in ADP-mediated platelet reactivity and thrombogenic biomarkers. vWF:Ag: von Willebrand factor antigen, ADP: adenosine diphosphate.

Table 1
Baseline clinical and laboratory characteristics

Values are presented as mean±standard deviation or number (%). *A p values are comparison between 3 test groups. CLPD: clopidogrel, CILO: cilostazol, PUFA: omega-3 polyunsaturated fatty acid, BMI: body mass index, LVEF: left ventricular ejection fraction, LAVI: left atrial volume index, GFR: glomerular filtration rate by modification of diet in renal disease equation, CVA: cerebrovascular accident, LDL-c: low-density lipoprotein cholesterol
Table 2
Thrombogenic biomarker levels during antiplatelet therapy
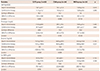
Table 3
Platelet reactivity during antiplatelet therapy

Table 4
Thrombogenic markers and platelet reactivity during aspirin monotherapy

Acknowledgments
This study was supported by a grant from Kunil Pharmacy and the Institute of Health Sciences, Gyeongsang National University. Kunil Pharmacy and Otsuka Korea provided the omega-3 polyunsaturated fatty acid (Omacor®) and cilostazol (Pletaal®), respectively.
References
1. Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009; 373:155–166.
2. Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008; 359:938–949.
3. McFadyen JD, Jackson SP. Differentiating haemostasis from thrombosis for therapeutic benefit. Thromb Haemost. 2013; 110:859–867.
4. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016; 37:2893–2962.
5. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. Circulation. 2014; 130:2071–2104.
6. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014; 383:955–962.
7. Bassand JP, Accetta G, Camm AJ, et al. Two-year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Eur Heart J. 2016; 37:2882–2889.
8. Son MK, Lim NK, Cho MC, Park HY. Incidence and risk factors for atrial fibrillation in Korea: the national health insurance service database (2002–2010). Korean Circ J. 2016; 46:515–521.
9. Sabir I, Khavandi K, Brownrigg J, Camm AJ. Oral anticoagulants for Asian patients with atrial fibrillation. Nat Rev Cardiol. 2014; 11:290–303.
10. Suzuki S, Yamashita T, Kato T, et al. Incidence of major bleeding complication of warfarin therapy in Japanese patients with atrial fibrillation. Circ J. 2007; 71:761–765.
11. Oh S, Goto S, Accetta G, et al. Vitamin K antagonist control in patients with atrial fibrillation in Asia compared with other regions of the world: real-world data from the GARFIELD-AF registry. Int J Cardiol. 2016; 223:543–547.
12. Choi JH, Cha JK, Huh JT. Adenosine diphosphate-induced platelet aggregation might contribute to poor outcomes in atrial fibrillation-related ischemic stroke. J Stroke Cerebrovasc Dis. 2014; 23:e215–e220.
13. Järemo P, Eriksson M, Lindahl TL, Nilsson S, Milovanovic M. Platelets and acute cerebral infarction. Platelets. 2013; 24:407–411.
14. ACTIVE Investigators. Connolly SJ, Pogue J, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009; 360:2066–2078.
15. Kim D, Lee SH, Kim BJ, et al. Secondary prevention by stroke subtype: a nationwide follow-up study in 46108 patients after acute ischaemic stroke. Eur Heart J. 2013; 34:2760–2767.
16. Shinohara Y, Katayama Y, Uchiyama S, et al. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 2010; 9:959–968.
17. Chowdhury R, Stevens S, Gorman D, et al. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis. BMJ. 2012; 345:e6698.
18. Larson MK, Ashmore JH, Harris KA, et al. Effects of omega-3 acid ethyl esters and aspirin, alone and in combination, on platelet function in healthy subjects. Thromb Haemost. 2008; 100:634–641.
19. Lee JH, Cha JK, Lee SJ, Ha SW, Kwon SU. Addition of cilostazol reduces biological aspirin resistance in aspirin users with ischaemic stroke: a double-blind randomized clinical trial. Eur J Neurol. 2010; 17:434–442.
20. Moshfegh K, Redondo M, Julmy F, et al. Antiplatelet effects of clopidogrel compared with aspirin after myocardial infarction: enhanced inhibitory effects of combination therapy. J Am Coll Cardiol. 2000; 36:699–705.
21. Kim IS, Jeong YH, Kang MK. Correlation of high post-treatment platelet reactivity assessed by light transmittance aggregometry and the VerifyNow P2Y12 assay. J Thromb Thrombolysis. 2010; 30:486–495.
22. ACTIVE Writing Group of the ACTIVE Investigators. Connolly S, Pogue J, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006; 367:1903–1912.
23. Siegbahn A, Oldgren J, Andersson U, et al. D-dimer and factor VIIa in atrial fibrillation - prognostic values for cardiovascular events and effects of anticoagulation therapy. A RE-LY substudy. Thromb Haemost. 2016; 115:921–930.
24. Wu N, Chen X, Cai T, et al. Association of inflammatory and hemostatic markers with stroke and thromboembolic events in atrial fibrillation: a systematic review and meta-analysis. Can J Cardiol. 2015; 31:278–286.
25. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010; 137:263–272.
26. Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013; 381:1107–1115.
27. Park Y, Franchi F, Rollini F, Angiolillo DJ. Update on oral antithrombotic therapy for secondary prevention following non-ST segment elevation myocardial infarction. Trends Cardiovasc Med. 2016; 26:321–334.
28. Hori M, Connolly SJ, Zhu J, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke. 2013; 44:1891–1896.
29. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365:883–891.
30. Hori M, Matsumoto M, Tanahashi N, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation - the J-ROCKET AF study -. Circ J. 2012; 76:2104–2111.
Supplementary Materials
The online-only Data Supplements are available with article at https://doi.org/10.4070/kcj.2016.0384.
Supplementary Table 2
Correlation between the changes in thrombogenic markers and platelet re Supplementary Table 1 activity in patients of CHA2DS2-VASc score ≥2




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download