Abstract
Background and Objectives
Palpitations are the most common complaint in cardiology outpatient clinics. These palpitations may be derived from paroxysmal atrial fibrillation (AF) and can be easily overlooked. It is unclear whether inter-atrial conduction time (ICT) may predict the paroxysmal AF in out-patients that present with palpitations. We evaluated the ability of the ICT to predict paroxysmal AF in these patients.
Subjects and Methods
The study group consisted of 199 patients (110 female). All patients underwent 24-hour Holter electrocardiogram (ECG) monitoring (total of 327 Holter ECG monitorings, mean: 1.64 times per patient). Brief episodes of AF were documented in 35 patients (20 female, Group 1). The remaining patients without AF were designated as Group 2 (90 female). All patients underwent routine transthoracic echocardiographic examination. ICT was also measured by echocardiography.
Results
The mean age in Group 1 was greater than in Group 2 (63.02±14 vs. 51.27±16 years, p<0.001). The left atrium was larger in Group 1 than in Group 2 (39.7±3.4 vs. 37.29±4.3 mm, p<0.001). The other echocardiographic variables were comparable between the two groups. The mean ICT was significantly higher in Group 1 than in Group 2 (138±14 vs. 114±12 msn, p<0.001). The ICT of 127 ms was predictive for AF with a sensitivity of 86% and specificity of 87% (p<0.001).
Conclusion
Our findings show that ICT was prolonged in patients with palpitations who developed a brief episode of AF in Holter ECG monitoring. ICT prolongation may be used to identify patients with palpitation that are at risk of an AF episode. Holter ECG monitoring should be repeated to detect AF episodes in patients with prolonged ICT.
Palpitations are the most common complaint in cardiology outpatient clinics. However, the majority of patients do not have evidence of palpitations, based on the electrocardiography (ECG) and Holter ECG monitoring. Patients who have sinus tachycardia, atrial fibrillation (AF) or other types of arrhythmia may also feel palpitations.1) AF incidence increases with age and can be responsible for up to one-third of ischemic strokes. It is also associated with silent cerebral infarctions and transient ischemic attacks.2) The self-terminating and often asymptomatic nature of paroxysmal AF may lead to under-diagnosis.
Strategies for AF detection include in-hospital ECG monitoring, serial ECG, Holter ECG monitoring, monitoring with the use of external event or loop recorders, long-term out-patient monitoring, and monitoring by with insertable cardiac monitors. A continuous and long term heart rhythm monitoring can be useful in unmasking AF episodes but is not feasible for most patients, and some of these procedures can be invasive.3)
The mechanism of AF is still currently debated. The prolongation of conduction time and shortened refractory period can increase susceptibility to AF attacks. Recurrence of AF is more common in patients with atrial conduction delay.4) Reports have also indicated that inter-atrial conduction time (ICT) prolongation could be associated with paroxysmal AF.5) It is unclear whether ICT could accurately predict silent AF in out-patients that present with palpitations. In this study, we investigated the value of ICT using transthoracic echocardiography in patients that presented with palpitation in an out-patient clinic.
This study included 199 consecutive patients that presented with complaints of palpitations in the cardiology outpatient clinic between 1st January 2014 and 1st January 2015. Patients had no history of documented arrhythmias and had sinus rhythm. Their heart rates were within normal ranges. The exclusion criteria of the study were as follows: presence of previous coronary and valvular heart disease, use of antiarrhythmic drugs, hyperthyroidism, and anemia. Blood samples were collected for thyroid function and routine blood tests. Routine transthoracic echocardiography was performed on all patients enrolled in the study, and those with structural and valvular heart disease were excluded from the study. In addition, patients with left ventricular hypertrophy were also excluded from the study. Patients with hypertension or using antihypertensive medications were excluded because these medications can affect ICT. However, patients taking oral antidiabetic therapy were enrolled in the study.
The echocardiography platform in the study provided real-time, simultaneous display of ECG and Doppler echocardiograms of patients. We evaluated routine echocardiographic parameters after that ICT was measured. The beginning of the P wave was marked in modified lead I, in which the right arm electrode was attached on the top right of the manubrium and the left arm electrode in the fifth intercostal space at the right parasternal line (Lewis lead). The ends of the P waves were marked as the top of the A wave trace which was obtained from the left atrium free wall next to the mitral annulus with the tissue Doppler recording (Fig. 1).
Routine 24-hour Holter ECG records were taken from all patients enrolled in the study. When the patients felt palpitation, the recordings were repeated. Accordingly, 24-hour Holter ECG was performed 327 times in 199 patients. AF was described in the Holter records and the characteristics on an electrocardiogram included irregular R-R intervals, absence of distinct repeating P waves and irregular atrial activity. An episode of AF is defined as an event lasting greater than 30 seconds in duration.
This study was approved by our hospital review board and informed consent was obtained from each patient.
Continuous variables are presented as the mean±standard deviation, whereas dichotomous variables are described as the number and percentage. The differences among the two groups were compared using the chi-square test for categorical variables and Student's t tests or Mann Whitney U test for continuous variables. An ICT cutoff point that predicted AF was calculated using receiver operating curve (ROC) analysis, and the sensitivity and specificity of this approach were estimated. Statistical analysis was performed using the Statistical Package for Social Sciences, version 16 (SPSS Inc., Chicago, IL, USA). A p value<0.05 was considered to indicate statistical significance.
This study included 199 (110 female, 55%) consecutively-enrolled patients with palpitations. AF was documented in 35 of all patients with 24-hour Holter ECG monitoring (Group 1). The remaining 164 patients that did not experience AF were placed in Group 2. Accordingly, 24-hour Holter ECG was performed 327 times in 199 patients (Group 1; 57 times, Group 2; 270 times, mean: 1.64 times per patient). In Group 2, 3 patients had regular nonsustained tachycardia attacks with narrow QRS complexes and 8 had rare ventricular ectopic beats.
Group 1 patients were older than the patients in Group 2 (63.02±14 vs. 51.27±16 years, p<0.001). There was no difference in gender frequency between the two groups. Similarly, the percentage of diabetes mellitus was comparable in the two groups (Table 1). Left ventricular ejection fraction was also similar in the two groups. However, LA diameter was significantly larger in Group 1 than in Group 2 (39.7±3.4 vs. 37.3±4.3 mm, p<0.001).
The ICT was measured for each patient after the routine echocardiography examination. ICT was significantly longer in Group 1 than in Group 2 (138±14 vs. 114±12 ms, p<0.001). Based on the ROC analysis, a cutoff point of 127 ms for ICT was identified with AF episodes with a sensitivity of 86% and specificity of 87% in patients with palpitations (p<0.001, Fig. 2).
In this study, we determined that ICT was more prolonged in patients with palpitations who developed a brief episode of AF, based on Holter ECG monitoring, compared with those who had no documented AF. A cutoff point of 127 ms for ICT identified AF episodes with a sensitivity of 86% and specificity of 87% in these patients.
Palpitation is an important complaint in cardiology outpatient clinics. However, patients do not typically have any ECG recorded at the time of their complaints. In addition, extended ECG records may remain unsuccessful in detecting arrhythmias in this population. In patients with palpitations with no history of cardiovascular disease, this situation sometimes remains unresolved.
In patients with implanted devices, silent AF episodes can easily be detected.6)7) The CRYptogenic STroke and underlying AtriaL Fibrillation (CRYSTAL AF) study showed that AF was more frequent in patients with a recent cryptogenic stroke (CS) who had insertable cardiac monitors that record these incidences more than standard follow-up care.8) It is very difficult to show the AF episodes without long-term monitoring. Our study results may reflect a practical approach for this problem. In the 30-Day Cardiac Event Monitor Belt For Recording Atrial Fibrillation After A Cerebral Ischemic Event (EMBRACE) trial, premature atrial beats were found to be associated with silent episodes of AF in cryptogenic emboli patients.9) Increased ICT is also associated with left atrial mechanical remodeling and dysfunction, both of which may be substrates for thromboembolism in CS patients.
The mechanism of AF is still currently being investigated. Patients with conduction time prolongation and shortened refractory period are more prone to developing AF attacks. This arrhythmia can occur if a suitable substrate exists in addition to the electrophysiological disorders mentioned.10) Interatrial conduction time can be measured with invasive methods in an electrophysiology laboratory. Electrophysiologically-measured ICT was correlated with ICT, which is calculated by transesophageal echocardiography.11) Kinay et al.12) have reported that ICT was associated with arrhythmia recurrence in patients with AF. Recent developments in imaging methods have increased the quality of transthoracic echocardiography, which have allowed the ICT to be measured. Fuenmayor et al.13) simultaneously measured the time interval between the electrocardiographic P wave and the mitral “a” wave using transthoracic Doppler echocardiography, and compared this noninvasive ICT with the invasive ICT measurement. They found that there was a significant correlation between the two methods.
AF is responsible for up to one-third of ischemic strokes and is associated with silent cerebral infarctions and transient ischemic attacks. ICT was significantly higher in cryptogenic ischemic stroke patients than in normal populations. A study by Karaca et al.14) indicated that prolonged ICT is a risk factor for stroke. In addition, ICT prolongation may predispose patients for developing AF. Lee et al.15) have reported that prolonged ICT (atrial electromechanical delays measurements) were better than the left atrial volume index and P wave amplitude for determining paroxysmal AF patients from the controls. Karaca et al.16) also investigated the relationship between ICT in postoperative AF in patients who underwent open heart surgical procedures; the authors they found that postoperative AF was less frequent in patients with longer ICT. Prolonged ICT is an important risk factor for AF, following coronary bypass operation. Based on this, ICT may be a potential marker for predicting patients that could experience AF episode.
In this study the AF patients were older and had a larger left atrium compared to patients in which this arrhythmia was not documented. It is expected that AF occurrence can increase with advanced age and with an enlarged left atrium.17) The increased atrial size would permit the coexistence of many reentrant circuits that could predispose patients for developing the AF.18) ICT, which can be easily measured with echocardiography, was found to be longer in the AF group, and intermittent AF attacks can be diagnosed with long and persistent ECG monitoring in long ICT patients.
There were some limitations to our study. The number of study subjects was small and the results may not be generalizable to larger populations. It is currently unclear whether prolongation of ICT can predict intermittent AF and additional research is needed on this approach in outpatient clinic patients. Therefore, further studies with full monitoring and a larger study population are needed to address this question.
Correct diagnosis of intermittent AF is very important because it has treatment implications for appropriate pharmacotherapy, including anticoagulation. Monitoring is necessary, especially in patients with high thromboembolic risk, to prevent a stroke event. The results of this study indicate that prolonged ICT may be a surrogate marker for undetected AF.
This is especially noteworthy if the presence of AF would likely change the current therapeutic approach and provide additional diagnostic tools for this particular patient population. In addition, this technique can be applied widely and may allow clinicians a better approach for monitoring patients to diagnose silent AF.
Figures and Tables
Fig. 1
Inter-atrial conduction time is defined as the time between the beginning of the P wave in the surface ECG and the top of the A wave recorded from the tissue Doppler imaging from the left atrial free wall next to the mitral annulus. ECG: electrocardiogram.
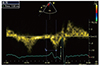
Fig. 2
Receiver operating curve of ICT for predicting atrial fibrillation. ICT: inter-atrial conduction time, AUC: area under the curve.

References
1. Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996; 100:138–148.
2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the framingham study. Stroke. 1991; 22:983–988.
3. de Asmundis C, Conte G, Sieira J, et al. Comparison of the patient-activated event recording system vs. traditional 24 h Holter electrocardiography in individuals with paroxysmal palpitations or dizziness. Europace. 2014; 16:1231–1235.
4. Papageorgiou P, Monahan K, Boyle NG, et al. Site-dependent intra-atrial conduction delay. Relationship to initiation of atrial fibrillation. Circulation. 1996; 94:384–389.
5. Cozma D, Mornos C, Pescariu S, Petrescu L, Lighezan D, Dragulescu SI. Electrophysiological and echocardiographic parameters predisposing to atrial fibrillation in patients with a structurally normal heart. Kardiol Pol. 2006; 64:143–150.
6. Lima C, Martinelli M, Peixoto GL, et al. Silent atrial fibrillation in elderly pacemaker users: a randomized trial using home monitoring. Ann Noninvasive Electrocardiol. 2016; 21:246–255.
7. Healey JS, Martin JL, Duncan A, et al. Pacemaker-detected atrial fibrillation in patients with pacemakers: prevalence, predictors, and current use of oral anticoagulation. Can J Cardiol. 2013; 29:224–228.
8. Sanna T, Diener HC, Passman RS. Crystal AF Steering Committee. Cryptogenic stroke and atrial fibrillation. N Engl J Med. 2014; 371:1261.
9. Gladstone DJ, Dorian P, Spring M, et al. Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke. 2015; 46:936–941.
10. Spach MS, Dolber PC, Heidlage JF. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation. Circ Res. 1988; 62:811–832.
11. Karaca M, Kinay O, Nazli C, Biceroglu S, Vatansever F, Ergene AO. The time interval from the initiation of the P-wave to the start of left atrial appendage ejection flow: does it reflect interatrial conduction time? Echocardiography. 2007; 24:810–815.
12. Kinay O, Nazli C, Ergene O, et al. Time interval from the initiation of the electrocardiographic P wave to the start of left atrial appendage ejection flow: a novel method for predicting atrial fibrillation recurrence. J Am Soc Echocardiogr. 2002; 15:1479–1484.
13. Fuenmayor AJ, Ramírez L, Fuenmayor AM. Validation of inter-atrial conduction time measurement by means of echo-doppler. Arch Cardiol Mex. 2002; 72:125–128.
14. Karaca M, Aytekin D, Kırıs T, Koskderelioglu A, Gedizlioglu M. Cryptogenic ischemic stroke and silent atrial fibrillation: what is the relationship? Springerplus. 2016; 5:130.
15. Lee DH, Choi SY, Park JS, et al. Comparison of prolonged atrial electromechanical delays with different definitions in the discrimination of patients with non-valvular paroxysmal atrial fibrillation. Korean Circ J. 2015; 45:479–485.
16. Karaca M, Demirbas MI, Biceroglu S, et al. Prediction of early postoperative atrial fibrillation after cardiac surgery: is it possible? Cardiovasc J Afr. 2012; 23:34–36.
17. Chei CL, Raman P, Ching CK, et al. Prevalence and risk factors of atrial fibrillation in chinese elderly: results from the Chinese longitudinal healthy longevity survey. Chin Med J (Engl). 2015; 128:2426–2432.
18. Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995; 92:835–841.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download