Abstract
Background and Objectives
Left bundle branch block (LBBB) with left axis deviation (LAD) has a worse prognosis than LBBB with a normal axis, and myocardial dysfunction has been suggested as a cause of left axis deviation. This study investigated the prognostic significance of the QRS axis in patients with LBBB and analyzed its relationship with the amount of myocardial scarring.
Subjects and Methods
A total of 829 patients were diagnosed with LBBB at Seoul National University Hospital from October 2004 to June 2014. Of these, 314 who were asymptomatic and had no previous history of cardiac disease were included in the present study. Myocardial scarring was calculated using the Selvester QRS scoring system, and LAD was defined as a QRS axis between -180° and -30°.
Results
Of the total patients, 91 (29%) had LAD, and patients were followed for a median of 30 months. During follow-up, two patients were hospitalized for de novo heart failure, four had pacemaker implants, and 10 died. There was a significant inverse correlation between myocardial scar score and the QRS axis (r=-0.356, p<0.001). Patients with concomitant LAD had a higher rate of major cardiac adverse events compared with patients with a normal axis (5.5% vs. 1.3%, log-rank p=0.010); the prognostic value was attenuated in multivariable analysis (hazard ratio 4.117; 95% confidence interval 0.955-17.743; p=0.058).
The clinical implications of left axis deviation (LAD) in patients with left bundle branch block (LBBB) have not been clearly elucidated. Some studies have reported that patients with LAD do not have a significantly different prognosis compared with patients with a normal QRS axis,1)2)3) while others have shown that patients with LAD have a higher incidence of conduction abnormalities and a poor prognosis, including cardiovascular death.4)5)6) Regardless of the differences in prognosis, previous studies have proposed that diseased myocardium is a plausible mechanism for axis deviation.3)4)
The Selvester QRS scoring system evaluates myocardial scarring based on 12-lead electrocardiogram (ECG) readings and has been updated for extensive application: it can be used to evaluate patients with bundle branch block and also those with pacing rhythm.7)8)9) The 12-lead ECG has valuable clinical significance and has been validated with cardiac magnetic resonance imaging.10)11) Furthermore, since the 12-lead ECG is a widely used diagnostic tool that is highly accessible, the myocardial scar score derived from ECG may be valuable in clinic environments.
In this study, the association between the QRS axis and the myocardial scar score was investigated in patients with LBBB. In addition, the importance of a left axis deviation concomitant with LBBB was evaluated.
This was a retrospective, observational cohort study. From October 2004 to June 2014, 829 patients were evaluated by 12-lead ECG and diagnosed with LBBB at Seoul National University Hospital (Seoul, Korea). A total of 314 of these patients were evaluated in the study. One hundred five patients were excluded because they did not have ECG results available for review, or they had been misdiagnosed with LBBB. An additional 410 patients were excluded due to one or more of the following: severe valvular heart disease, presence of an implantable cardiac defibrillator or cardiac resynchronization therapy (CRT) device, history of cardiac surgery or intervention, ischemic heart disease documented by imaging studies, symptoms of ischemic heart disease or heart failure, or previously proven cardiomyopathy or myocarditis (Fig. 1).
We obtained demographic data, data on the presence of underlying diseases, clinical presentation, laboratory test results, and the initial diagnosis of LBBB from the hospital electronic medical records. Twelve-lead ECGs were performed as routine practice. Blood sampling and tests were performed as part of routine practice by laboratories certified by the Korean Association of Quality Assurance for Clinical Laboratory. The study protocol was approved by our Institutional Review Board.
The criteria for diagnosing LBBB by 12-lead ECG were defined as: QR or RS in leads V1 or V2; mid-QRS notching or slurring in ≥2 of leads V1, V2, V5, V6, I, and aVL; and QRS duration ≥140 ms (male) or 130 ms (female).12)13) Normal axis was defined as -30° ≦R axis <90°. In addition to information on gender and age, a myocardial scar score was calculated according to the previously reported Selvester scoring protocol, which was applicable for LBBB patients.8)10)
The primary endpoint was any major adverse cardiovascular event (MACE), defined as a composite of sustained ventricular tachycardia or ventricular fibrillation, pacemaker implantation due to complete atrioventricular block, CRT implantation, hospital admission due to heart failure, or cardiovascular death.
Data are described as mean±standard deviation for continuous variables and as numbers and frequencies for categorical variables. The χ2 test or Fisher's exact test was applied for categorical variables, and an unpaired Student's t-test was used for continuous variables. A receiver-operating-characteristics curve was used to evaluate the predictive power of the myocardial scar score on LAD. Kaplan-Meier estimates were applied to evaluate the chronological inclination of outcomes, and the log-rank test was used to analyze the differences between groups (subjects with LAD, subjects with normal axis). A multivariable Cox proportional-hazards regression model was used to investigate the independent prognostic indicators of MACE. Variables associated with MACE with p<0.1 in the univariate analysis were included as confounding factors in the multivariate analysis. A two-sided p<0.05 was considered statistically significant. Statistical tests were performed using IBM SPSS Statistics version 22 (SPSS Inc., Chicago, IL, USA).
A total of 314 patients diagnosed with LBBB met the criteria for analysis. Baseline characteristics were presented in Table 1. The mean age was 68 years, 105 patients (33%) were male, 107 patients (34%) had hypertension, 89 patients (28%) had diabetes mellitus, and 32 patients (10%) had dyslipidemia. When classified according to QRS axis, a total of 91 patients (29%) were classified with LAD, and the remaining 71% had a normal axis. The mean±standard deviation myocardial scar score of all LBBB patients was 3.99±2.12 at baseline. The mean±standard deviation myocardial scar score was 3.6±2.0 for the normal axis group and 5.1±2.0 for the LAD group. The LBBB patients with normal QRS axis were younger and had comparable prevalence of hypertension, diabetes mellitus, and dyslipidemia to those with left axis deviation.
There was a significant inverse correlation between the myocardial scar score and the QRS axis (r=-0.356, p<0.001) (Fig. 2A). The myocardial scar score was also significantly associated with age (r=0.184, p=0.001) and hemoglobin level (r=-0.165, p=0.007); it was not correlated with body mass index (r=-0.028, p=0.627), total cholesterol level (r=-0.039, p=0.526), or serum creatinine level (r=0.040, p=0.504). In the ROC curve analysis, the area under the curve of the myocardial scar score to predict LAD was 0.700 (95% CI 0.639-0.761, p<0.001) (Fig. 2B).
The median follow-up duration was 30 months (interquartile range: 6 to 63 months). During the follow-up period, 10 of 314 patients died (including two cardiovascular deaths caused by ventricular fibrillation). Four patients underwent pacemaker implantation due to complete atrioventricular block, and two patients were admitted to the hospital due to de novo acute heart failure (Table 2). None of the patients underwent CRT insertion. Stratification according to the QRS axis indicated that patients in the LAD group were older and demonstrated a higher incidence of MACE compared with patients with a normal axis (5.5% vs. 1.3%, log-rank p=0.010) (Fig. 3).
In a Cox-proportional hazard regression analysis, LAD was a significant prognostic indicator of MACE in the univariate analysis [hazard ratio (HR) 5.400; 95% confidence interval (CI) 1.281-22.758; p=0.022], while myocardial scar score failed to show prognostic implication (HR 0.877; 95% CI 0.619-1.243; p=0.460). The LAD predictive value was marginally attenuated in multivariate analysis (HR 4.117; 95% CI 0.955-17.743, p=0.058) after adjusting for the confounding variables of age, sex, and diabetes mellitus (Table 3).
The study analysis indicated that the QRS axis of LBBB patients was associated with the myocardial scar score, and LAD concomitant with LBBB was a prognostic indicator of MACE. The clinical prognostic importance of LAD in LBBB patients has been reported in numerous studies; however, the results remain controversial.1)2)3)4)5)6)14)15) A recent study designed to include subjects without clinical cardiovascular disease reported that LAD was associated with a higher risk of mortality and cardiovascular disease regardless of concomitant LBBB.16) The reason for the different prognostic impact between studies, including ours, may be explained by the different study populations, definition of LAD, and study period (Table 4). Although the exact mechanism for developing LAD is still uncertain, it has been suggested that comorbidities such as hypertension and diabetes mellitus exert an influence and result in conduction disturbance of fascicles and the main left bundle.3)16) In addition to prolonged and asynchronous contraction due to concomitant LBBB, this could also cause poor clinical outcomes.
Myocardial scarring is generated by both ischemic and nonischemic damage.17)18)19) The electrical depolarization initiates from the free and septal walls of the right ventricle in LBBB patients, but this vector may be interrupted and recorded differently in an ECG if myocardial scarring is present.8) Because myocardial scarring is considered to be a cumulative indicator reflecting myocardial damage, we speculated that the QRS axis is related to the myocardial scar score.
The Selvester QRS score, a quantitative scoring system calculated from 12-lead ECG, can be used to estimate the myocardial scar amount in the left ventricle. The scoring system was established to estimate the myocardial infarct size and has been improved and updated for applications in various conditions, including LBBB.7)8)9)10) In LBBB patients, the myocardial scar calculated by the Selvester QRS score and that measured by cardiac magnetic resonance imaging were significantly correlated (r=0.80, p<0.001) and showed a mean difference of 7.6% overestimation. In regard to reproducibility, absolute differences of 0.4 and 0.6 myocardial scar scores were observed in intraobserver and interobserver examination, respectively. The intraobserver and interobserver agreement were κ=0.96 and κ=0.86, respectively, in a previous report.10) In this study, we measured the myocardial scar with the Selvester QRS scoring system based on the accuracy and reproducibility of the test.
Our data was similar to previous studies that investigated the prognosis of patients with LBBB and LAD and demonstrated a significant negative correlation between the myocardial scar score and the QRS axis in patients with LBBB. Despite the significant inverse relationship between myocardial scar score and QRS axis, a high myocardial scar score was not a statistically significant prognostic indicator, in contrast to the presence of LAD in LBBB patients (Table 3). Results have indicated that LAD, not the myocardial scar score, was a prognostic indicator in LBBB patients, and that LAD might be partially influenced by myocardial scarring as well as by other confounding pathologic factors.
There were several limitations to our analysis. This was a retrospective cohort study, rather than a prospective cohort study; therefore, there could be unmeasured confounding variables that influenced the results. Additionally, although we only included asymptomatic patients who did not have abnormal cardiac imaging studies, we were not able to completely eliminate patients had asymptomatic structural or functional heart disease who therefore did not undergo a cardiac evaluation before the recruitment. Finally, the R/S or R/Q ratio at lead II was included in the Selvester QRS scoring system, suggesting that patients with LAD had higher myocardial scar scores. However, the myocardial scar score difference between the two groups was larger than the scoring point of lead II.
Figures and Tables
Fig. 1
Flow chart of this study. LBBB: left bundle branch block, ECG: electrocardiogram, ICD: implantable cardioverter defibrillator, CRT: cardiac resynchronization therapy, MACE: major adverse cardiac event.

Fig. 2
Association between QRS axis and myocardial scar score. (A) There was an inverse correlation between QRS axis and myocardial scar score. (B) Receiver-operating curves analysis: area under the curve of myocardial scar score to predict left axis deviation.

Fig. 3
Kaplan-Meier curves of major adverse cardiovascular event incidence according to QRS axis in left bundle branch block patients.
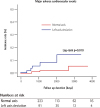
Table 1
Baseline characteristics stratified by QRS axis

Table 2
Major adverse cardiovascular events according to QRS axis

Table 3
Univariate and multivariate Cox regression analyses for major adverse cardiac events

Table 4
Comparison of studies about prognosis of left axis deviation in LBBB patients

References
1. Haft JI, Herman MV, Gorlin R. Left bundle branch block: etiologic, hemodynamic, and ventriculographic considerations. Circulation. 1971; 43:279–287.
2. Yano K, Peskoe SM, Rhoads GG, Moore JO, Kagan A. Left axis deviation and left anterior hemiblock among 8,000 Japanese-American men. Am J Cardiol. 1975; 35:809–815.
3. Patel PJ, Verdino RJ. Usefulness of QRS axis change to predict mortality in patients with left bundle branch block. Am J Cardiol. 2013; 112:390–394.
4. Dhingra RC, Amat-Y-Leon F, Wyndham C, Sridhar SS, Wu D, Rosen KM. Significance of left axis deviation in patients with chronic left bundle branch block. Am J Cardiol. 1978; 42:551–556.
5. Lichstein E, Mahapatra R, Gupta PK, Chadda KD. Significance of complete left bundle branch block with left axis deviation. Am J Cardiol. 1979; 44:239–242.
6. Parharidis G, Nouskas J, Efthimiadis G, et al. Complete left bundle branch block with left QRS axis deviation: defining its clinical importance. Acta Cardiol. 1997; 52:295–303.
7. Wagner GS, Freye CJ, Palmeri ST, et al. Evaluation of a QRS scoring system for estimating myocardial infarct size. I. Specificity and observer agreement. Circulation. 1982; 65:342–347.
8. Strauss DG, Selvester RH. The QRS complex--a biomarker that “images” the heart: QRS scores to quantify myocardial scar in the presence of normal and abnormal ventricular conduction. J Electrocardiol. 2009; 42:85–96.
9. Lee SA, Cha MJ, Cho Y, Oh IY, Choi EK, Oh S. Paced QRS duration and myocardial scar amount: predictors of long-term outcome of right ventricular apical pacing. Heart Vessels. 2016; 31:1131–1139.
10. Strauss DG, Selvester RH, Lima JA, et al. ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol. 2008; 1:327–336.
11. Strauss DG, Cardoso S, Lima JA, Rochitte CE, Wu KC. ECG scar quantification correlates with cardiac magnetic resonance scar size and prognostic factors in Chagas' disease. Heart. 2011; 97:357–361.
12. Loring Z, Chelliah S, Selvester RH, Wagner G, Strauss DG. A detailed guide for quantification of myocardial scar with the Selvester QRS score in the presence of electrocardiogram confounders. J Electrocardiol. 2011; 44:544–554.
13. Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011; 107:927–934.
14. Pryor R, Blount SG Jr. The clinical significance of true left axis deviation. Left intraventricular blocks. Am Heart J. 1966; 72:391–413.
15. Beach TB, Gracey JG, Peter RH, Grunenwald PW. Benign left bundle branch block. Ann Intern Med. 1969; 70:269–276.
16. Miller WL, Hodge DO, Hammill SC. Association of uncomplicated electrocardiographic conduction blocks with subsequent cardiac morbidity in a community-based population (Olmsted County, Minnesota). Am J Cardiol. 2008; 101:102–106.
17. McCrohon JA, Moon JC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003; 108:54–59.
18. Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003; 41:1561–1567.
19. Blyth KG, Groenning BA, Martin TN, et al. Contrast enhanced-cardiovascular magnetic resonance imaging in patients with pulmonary hypertension. Eur Heart J. 2005; 26:1993–1999.
20. Davis JA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc. 1985; 17:6–21.
21. Gaultier C, Boule M, Thibert M, Leca F. Resting lung function in children after repair of tetralogy of Fallot. Chest. 1986; 89:561–567.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download