Abstract
Background and Objectives
In clinical trials, hypertensive patients tend to have higher interleukin-18 (IL-18) concentrations than normotensive groups, but the relationship between IL-18 and left ventricular hypertrophy (LVH), which is a marker of end-organ damage, is not well studied. We aimed to investigate the relationship between IL-18 and LVH in apparently healthy subjects free of clinically significant atherosclerotic disease.
Subjects and Methods
We enrolled 198 subjects (102 women and 96 men) between May 2006 and March 2007, who were free of cardiovascular or immune diseases, but were suspected to have hypertension. Twenty-four-hour ambulatory blood pressure monitoring and two-dimensional echocardiography were performed. Lipid profiles, high-sensitivity CRP (hs-CRP), IL-18, and whole blood cell counts were measured for all subjects.
Results
White blood cell count, hs-CRP, left ventricular mass, left ventricular mass index (LVMI), and IL-18 were higher in the hypertensive group than in the normotensive group (p=0.045, p=0.004, p<0.0001, p=0.001, and p=0.017 respectively). Twenty-four hour day and night systolic and diastolic blood pressure averages were positively correlated with IL-18 level in the entire study population. In multivariate regression analysis, left ventricular mass index and hs-CRP level were independently associated with IL-18 level in both the hypertensive group and the entire study population (β=0.154, β=0.149 p=0.033, p=0.040 and β=0.151, β=0.155 p=0.036, p=0.032 respectively)
The relationship between inflammation and hypertension has been investigated for many years.1) Experimental studies have shown that inflammatory cytokines contribute to hypertension by increasing vascular tone and impairing endothelial function. The relationships between hypertension and high-sensitivity CRP (hs-CRP), tumor necrosis factor-α, interleukin (IL)-6, and IL-18 are well known,2) but the relationship between IL-18 and left ventricular hypertrophy (LVH) is not well studied.
Inflammatory cells are activated during acute coronary syndromes, and some indexes such as the neutrophil to lymphocyte ratio3) and the white blood cell (WBC) count to mean platelet volume4) ratio are associated with worse prognosis. Like inflammatory cells, IL-18 was elevated in acute coronary syndromes, and high levels were correlated with worse prognosis.5) In addition, the role of IL-18 in carotid intima media thickness (a marker of subclinical atherosclerosis) is well established.6) In experimental animal studies, there is growing evidence regarding the relationship aming IL-18, hypertension, and LVH. IL-18 knockout mice subjected to pressure overload developed less hypertrophy, but also had worse contractile function.7) Daily administration of IL-18 in healthy mice induces myocardial hypertrophy and contractile dysfunction.8) These results suggest that IL-18 is involved in the hypertrophic response in overload cardiomyopathy.
In clinical trials, hypertensive patients tend to have higher IL-18 concentrations than normotensive groups,9)10) but the relationship between IL-18 and LVH, which is a marker of end-organ damage, is unknown in humans. In this study, we aimed to investigate the relationship between IL-18 and left ventricular mass (LVM) in apparently healthy subjects with or without hypertension who were free of atherosclerotic disease.
We performed an observational, cross-sectional study in order to examine the relationships between inflammation, hypertension, and LVH. The study population was a sample of subjects admitted to the cardiology department of our institute and who were suspected to have hypertension (n=198). Subjects were eligible to participate in the study if they were 20 to 60 years of age, had no history of hypertension or cardiovascular (CV) disease (coronary artery disease, stroke, peripheral vascular disease), were not using antihypertensive medications, were free of any other major systemic illnesses, and were not pregnant. We performed electrocardiogram and a treadmill test to rule out clinically significant coronary artery disease. A positive or non-diagnostic test result according to the modified Bruce protocol was considered an exclusion criterion. We recruited 198 subjects (102 women, 96 men) who met these criteria. The local ethics committee assessed and approved the study, and written informed consent for participation in the study was obtained from all individuals
Whole blood count, total, high-density lipoprotein, and low-density cholesterol, triglycerides, glucose, and serum hs-CRP and IL-18 concentrations were measured using commercially available enzyme-linked immunosorbent assay (Diagnostic Products Corporation, Los Angeles, CA, USA). Electrocardiogram and two-dimensional echocardiography were performed in all of the subjects participating in the study. The Sokolow-Lyon index (SLI) was calculated as the sum of the amplitudes of V1S and V5R.
Echocardiographic studies were performed using the Acuson Sequoia C256 (Siemens Medical Solutions USA Inc., Mountain View, CA, USA) or the SONOS 7500 (Phillips, Andover, MA, USA). All echocardiographic measurements and the estimation of LV mass were performed according to the recommendations of the American Society of Echocardiography and the European Association of Echocardiography.11)
The 24 hour ambulatory blood pressure (BP) monitoring was performed with oscillometric equipment (Tonoport GE, Berlin, Germany). The cuff was applied to the non-dominant arm at the end of the visit. The device was set to obtain ambulatory BP and heart rate readings at 30 minutes intervals for 24 hours, during which the subjects were sent home and asked to perform their usual activities. The subjects were asked to keep a diary of daily activities and the time of sleep and to return to the hospital 24 hours later. Hypertension was determined based on 24 hour systolic and diastolic BP averages; a 130/80 mmHg cut-off value was used.
Statistical calculations were performed using SPSS 15 for Windows (SPSS Inc, Chicago, IL, USA). Continuous and normally distributed variables are presented as mean±standard deviation, and non-normally distributed variables are presented as median (min-max). Categorical data is presented as frequency and percentage. Continuous variables were compared among the groups of patients with student's t-test for normally distributed data and the Kruskal–Wallis test for non-normally distributed data. The Chi-square test was used for comparing categorical variables among groups. The Pearson test for normally distributed data and the Spearman test for non-normally distributed data were used for bivariate correlations. Logistic and linear regression was used for multivariate analysis. All tests were two-tailed, and a p<0.05 was considered significant.
The study population was composed of 102 women and 96 men between the ages of 20-60 years. Patients were divided into hypertensive (n=76) and normotensive (n=120) groups according to average 24 hour BP value.
The 24 hour systolic and diastolic BP averages and day and night systolic and diastolic BP averages were significantly higher in the hypertensive group. Gender and age were similar between groups. The frequency of type 2 diabetes was very low in the study population (2.5%) and was not statistically different between groups. Average body mass index was higher in the hypertensive group than the normotensive group. LVM, left ventricular mass index (LVMI), SLI, WBC count, hs-CRP, and IL-18 were higher in the hypertensive group than in the normotensive group (Table 1).
Day, night, and 24 hour systolic and diastolic blood pressure averages were all correlated with IL-18 level in the total study population. We also found positive correlations between IL-18 and body mass index (BMI) LVM, LVMI, triglyceride, and hs-CRP and a negative correlation between IL-18 and high-density lipoprotein (Table 2). The correlation between IL-18 and 24 hour systolic BP are shown in Fig. 1, and the correlation between IL-18 and LVMI is shown in Fig. 2.
In the hypertensive group, only night systolic BP average and LVM were correlated with IL-18 level (Table 3). In the normotensive group, no significant correlations were found between BP averages and IL-18; however, BMI, LVM, and hs-CRP level were all positively correlated with IL-18 (Table 4).
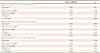
In multivariate analysis, the correlations between IL-18, LVMI, and hs-CRP remained statistically significant in the entire study population. Similar relationships were found in the hypertensive group. In contrast to these findings, we did not find any independent relationships in normotensive patients. On multivariate analysis, there were no statistically significant relationships between IL-18 level and BP parameters (Table 5). Therefore, IL-18 level was independently associated with LVMI and hs-CRP level in the whole study population and in newly diagnosed hypertensive patients.
We found that hypertensive patients had higher IL-18 serum level than normotensive subjects. BP averages were also positively correlated with serum IL-18 level in the entire study population. IL-18 level was independently associated with LVMI and hs-CRP level in the whole study population and in newly diagnosed hypertensive patients. We did not find any independent relationships between IL-18 and LVMI in normotensive subjects. Normotensive subjects do not have clinically significant CV disease or hypertension, both of which are known to alter LVMI and IL-18 level. Therefore, the absence of these diseases may explain the lack of any relationship between these parameters in this group. We hypothesize that the independent relationships seen in the general population were mainly due to the hypertensive subjects.
To our knowledge, our study is the first to show an independent relationship between LVMI and IL-18 level in humans. In most previous studies, hypertensive subjects had higher IL-18 level than normotensive subjects, and IL-18 level was positively correlated with office BP.9)10) These findings are consistent with our study findings. However, unlike out study, the PRIME study (n=1005) did not find a statistically significant difference in IL-18 level between hypertensive and normotensive groups.12) In that study, all participants were males between 50-59 years of age, and interleukin level was measured in plasma rather than serum. The narrow range of age and gender might have obscured the relationship between IL-18 and hypertension.
We used ambulatory blood pressure readings, which are more accurate and have different diagnostic criteria than office measurements.13) Several studies have shown that LVH and other markers of organ damage correlate with ambulatory BP more closely than with office BP in hypertensive patients.14) Evidence from meta-analyses of published observational studies and pooled individual data has shown that ambulatory BP is a more sensitive risk predictor of clinical CV outcomes, such as coronary morbid or fatal events and stroke, than office BP.15) The superiority of ambulatory BP has been shown in the general population, in hypertensive patients, in patients at high risk, and in patients with CV disease.16)17) These factors make our findings more valuable.
Left ventricular hypertrophy, especially of the concentric type, is associated with a CV disease risk higher than 20% in 10 years.18) In hypertensive patients with LVH, a therapeutically-induced reduction of LVM was significantly associated with CV event reduction.19) Antihypertensive treatment decreases the CV events associated with hypertension; however, despite successful treatment, risk remains higher than in the general population.20) This finding suggests that hypertension-related CV risk is associated with factors other than high BP. One of these factors might be inflammation; if so, hypertension treatments targeted to inflammation may result in additional risk reduction.
There are several mechanisms which may explain the relationship between inflammatory cytokines and hypertension. Increased vascular oxidative stress is one of the postulated unifying pathways that leads to hypertension. Vasoactive hormones that play an important role in the pathogenesis of hypertension (e.g., angiotensin II, endothelin I), growth factors (e.g., platelet-derived growth factor, transforming growth factor-β), and mechanical stimuli (e.g., shear stress and stretch) activate nicotinamide adenine dinucleotide phospate and result in increased levels of reactive oxygen species.21) Increased reactive oxygen species damage the vascular wall and impair vasodilation by inactivation of nitric oxide.22) Angiotensin II enhances the expression of IL-18 receptor in vascular smooth muscle cells.23) Angiotensin II, along with aldosterone and endothelin-1, increases IL-18 messenger ribonucleic acid (mRNA) and protein expression in cardiomyocytes.24)
The sympathetic nervous system plays an important role in the pathophysiology of hypertension, and evidence supports increased release of epinephrine from the hearts of patients with essential hypertension.25) Stimulation of the β2-adrenergic receptor activates IL-18 promoter activity, upregulates IL-18 mRNA, and increases IL-18 mRNA stability and IL-18 protein expression in endothelial cells.26)
Two strategies have been used to counter the effects of IL-18: IL-18 binding protein (IL-18BP), a naturally occurring protein, and a neutralizing IL-18 antibody. Recombinant human IL-18BP has been investigated in clinical trials and was found to be safe in healthy and obese volunteers27) and in patients with psoriasis and rheumatoid arthritis.28) Evidence from clinical and experimental data suggests that IL-18 may be a therapeutic target in acute myocardial infarction,29) but no trials have tested this hypothesis in acute myocardial infarction or HF. Likewise, no trials have investigated IL-18 blockade, LVH, and hypertension.
However, we showed that the relationship between IL-18 and LVMI is quite weak. Based on the scope of the accumulated data in the literature regarding IL-18 blockage as a therapeutic intervention, we hypothesize that therapies directed against the inflammatory system may eventually be an option.
Our study has several limitations including a relatively small sample size. Furthermore, the cross-sectional and observational nature of our study does not allow us to determine cause and effect relationships. The elimination half-life of IL-18 in vivo is approximately six hours.30) Blood samples were drawn early in the morning, so our measurements did not represent the entire day and might have missed any diurnal variations. This makes it difficult to interpret the relationship between IL-18 concentration and BP variation, particularly that occurring at times other than that at which the blood samples were drawn. For instance, variations occurring in the afternoon might have changed IL-18 level significantly, but we likely did not detect this effect due to the relatively short elimination half-life. Because of this study's observational and cross sectional design, we cannot make any suggestions about patient follow up or treatment strategies. Further studies with more participants should be performed to clarify these matters.
Our study showed that elevated level of IL-18 is associated with hypertension in newly diagnosed hypertensive patients and in the general population. We also found that LVMI was independently associated with IL-18 in both hypertensive patients and in the general population. These findings comprise a rationale for further larger scale studies that could reveal a cause and effect relationship among IL-18, hypertension, and related left ventricular hypertrophy. However, based on our results, it is too early to suggest any therapeutic interventions targeting IL-18 for treatment of hypertension and LVH. Stronger relaionships supported by future studies may lead to innovations in the treatment of hypertension and LVH in the coming decades.
Figures and Tables
Table 1
Comparison between hypertensive and normotensive groups

Values are presented as mean±standard deviation or number (%). Student's t-test and Kruskal Wallis tests were used. BMI: body mass index, DM: diabetes mellitus, BP: blood pressure, LVM: left ventricular mass, LVMI: left ventricular mass index, WBC: white blood cell, hs-CRP: high sensitivity C-reactive protein, IL-18: interleukin-18
Table 2
Bivariate correlations with IL-18 level in the entire study population
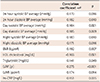
Table 3
Bivariate correlations with IL-18 level in the hypertensive group
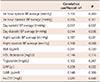
Table 4
Bivariate correlations with IL-18 level in the normotensive group

Table 5
Regression analysis of IL-18 level
References
1. Bautista LE, Lopez-Jaramillo P, Vera LM, Casas JP, Otero AP, Guaracao AI. Is C-reactive protein an independent risk factor for essential hypertension? J Hypertens. 2001; 19:857–861.
2. Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005; 19:149–154.
3. Borekci A, Gur M, Turkoglu C, et al. Neutrophil to lymphocyte ratio predicts left ventricular remodeling in patients with ST elevation myocardial infarction after primary percutaneous coronary intervention. Korean Circ J. 2016; 46:15–22.
4. Dehghani MR, Rezaei Y, Fakour S, Arjmand N. White blood cell count to mean platelet volume ratio is a prognostic factor in patients with non-ST elevation acute coronary syndrome with or without metabolic syndrome. Korean Circ J. 2016; 46:229–238.
5. Hulthe J, McPheat W, Samnegard A, Tornvall P, Hamsten A, Eriksson P. Plasma interleukin (IL)-18 concentrations is elevated in patients with previous myocardial infarction and related to severity of coronary atherosclerosis independently of C-reactive protein and IL-6. Atherosclerosis. 2006; 188:450–454.
6. Aso Y, Okumura K, Takebayashi K, Wakabayashi S, Inukai T. Relationships of plasma interleukin-18 concentrations to hyperhomocysteinemia and carotid intimal-media wall thickness in patients with type 2 diabetes. Diabetes Care. 2003; 26:2622–2627.
7. Colston JT, Boylston WH, Feldman MD, et al. Interleukin-18 knockout mice display maladaptive cardiac hypertrophy in response to pressure overload. Biochem Biophys Res Commun. 2007; 354:552–558.
8. Woldbaek PR, Sande JB, Stromme TA, et al. Daily administration of interleukin-18 causes myocardial dysfunction in healthy mice. Am J Physiol Heart Circ Physiol. 2005; 289:H708–H714.
9. Vilarrasa N, Vendrell J, Maravall J, et al. IL-18: relationship with anthropometry, body composition parameters, leptin and arterial hypertension. Horm Metab Res. 2006; 38:507–512.
10. Thorand B, Kolb H, Baumert J, et al. Elevated levels of interleukin-18 predict the development of type 2 diabetes: results from the MONICA/KORA Augsburg study, 1984-2002. Diabetes. 2005; 54:2932–2938.
11. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015; 28:1–39.e14.
12. Blankenberg S, Luc G, Ducimetiere P, et al. Interleukin-18 and the risk of coronary heart disease in European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME). Circulation. 2003; 108:2453–2459.
13. Pickering TG, White WB. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens. 2008; 2:119–124.
14. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008; 26:1919–1927.
15. Conen D, Bamberg F. Noninvasive 24-h ambulatory blood pressure and cardiovascular disease: a systematic review and meta-analysis. J Hypertens. 2008; 26:1290–1299.
16. Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007; 370:1219–1229.
17. Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008; 51:55–61.
18. Mancia G, Laurent S, Agabiti-Rosei E, et al. Reappraisal of European guidelines on hypertension management: a European society of hypertension task force document. J Hypertens. 2009; 27:2121–2158.
19. Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004; 292:2350–2356.
20. Blacher J, Evans A, Arveiler D, et al. Residual cardiovascular risk in treated hypertension and hyperlipidaemia: the PRIME study. J Hum Hypertens. 2010; 24:19–26.
21. Rabkin SW. The role of interleukin 18 in the pathogenesis of hypertension-induced vascular disease. Nat Clin Pract Cardiovasc Med. 2009; 6:192–199.
22. Clempus RE, Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res. 2006; 71:216–225.
23. Sahar S, Dwarakanath RS, Reddy MA, Lanting L, Todorov I, Natarajan R. Angiotensin II enhances interleukin-18 mediated inflammatory gene expression in vascular smooth muscle cells: a novel cross-talk in the pathogenesis of atherosclerosis. Circ Res. 2005; 96:1064–1071.
24. Doi T, Sakoda T, Akagami T, et al. Aldosterone induces interleukin-18 through endothelin-1, angiotensin II, Rho/Rho-kinase, and PPARs in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008; 295:H1279–H87.
25. Rumantir MS, Jennings GL, Lambert GW, Kaye DM, Seals DR, Esler MD. The ‘adrenaline hypothesis’ of hypertension revisited: evidence for adrenaline release from the heart of patients with essential hypertension. J Hypertens. 2000; 18:717–723.
26. Chandrasekar B, Marelli-Berg FM, Tone M, Bysani S, Prabhu SD, Murray DR. Beta-adrenergic stimulation induces interleukin-18 expression via beta2-AR, PI3K, Akt, IKK, and NF-kappaB. Biochem Biophys Res Commun. 2004; 319:304–311.
27. Mistry P, Reid J, Pouliquen I, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single-dose antiinterleukin-18 mAb GSK1070806 in healthy and obese subjects. Int J Clin Pharmacol Ther. 2014; 52:867–879.
28. Tak PP, Bacchi M, Bertolino M. Pharmacokinetics of IL-18 binding protein in healthy volunteers and subjects with rheumatoid arthritis or plaque psoriasis. Eur J Drug Metab Pharmacokinet. 2006; 31:109–116.
29. O'Brien LC, Mezzaroma E, Van Tassell BW, et al. Interleukin-18 as a therapeutic target in acute myocardial infarction and heart failure. Mol Med. 2014; 20:221–229.
30. Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schonbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002; 195:245–257.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download