Abstract
Background and Objectives
Vitamin D is generally known to be closely related to inflammation. The effects of vitamin D on coronary artery disease (CAD) are not fully explained. Nowadays, coronary artery ectasia (CAE) cases are common and are regarded as being a kind of CAD. We aimed to investigate, in a case-control study, the relationship between vitamin D and CAE without an associated inflammatory process.
Subjects and Methods
This study population included 201 patients (CAE group, 121 males; mean age, 61.2±6.4 years) with isolated CAE; and 197 healthy individuals (control group, 119 males; mean age, 62.4±5.8 years), comprising the control group, who had normal coronary arteries. These participants concurrently underwent routine biochemical tests, tests for inflammatory markers, and tests for 25-OH vitamin D in whole-blood draws. These parameters were compared.
Results
There are no statistical significance differences among the groups for basic clinical characteristics (p>0.05). Inflammatory markers were recorded and compared to exclude any inflammatory process. All of them were similar, and no statistical significance difference was found. The average parathyroid hormone (PTH) level of patients was higher than the average PTH level in controls (41.8±15.1 pg/mL vs. 19.1±5.81 pg/mL; p<0.001). Also, the average 25-OH vitamin D level of patients was lower than the average 25-OH vitamin D level of controls (14.5±6.3 ng/mL vs. 24.6±9.3 ng/mL; p<0.001). In receiver operating characteristic curve analysis, the observed cut-off value for vitamin D between the control group and patients was 10.8 and 85.6% sensitivity and 75.2% specificity (area under the curve: 0.854, 95% confidence interval: 0.678-0.863).
All vitamins are essential for life; however, the importance of vitamin D, has increased with recent studies.1) The reason for this is that it is associated with many chronic diseases and inflammatory processes.2) On the other hand, its levels were lower in these conditions. Naturally, vitamin D deficiency suggests various cardiovascular diseases such as coronary artery disease (CAD), heart failure, and hypertension (HT).2)3) According to various studies, these effects are being made via the renin angiotensin aldosterone system (RAAS). There is increasing evidence supporting the role of RAAS in aneurysm development.4)5)
Aortic aneurysms are related with mortality, and the causes of aneurysms are somewhat unknown.6) A variant of them is coronary artery ectasia (CAE). It is an abnormality of the coronary anatomy and has been characterised as a localized or diffuse non-obstructive lesion of the epicardial coronary arteries, by the enlargement of a diameter at least 1.5 times greater than the diameter of the normal portion of the artery.7) The prevalence of CAE has been found in up to 5% of patients undergoing coronary angiography.8)
In the literature, there is a similar study.9) However, as previously mentioned, vitamin D levels are already typically low in inflammatory processes. Nevertheless, chronic diseases involved in the inflammation process could not be excluded in that study. Also, the small number of their study subjects makes the generalizability of their results debatable. Thus, we sought to investigate vitamin D in terms of its being a novel marker for patients with CAE without any inflammatory processes-aiming to get rid of confusion regarding the treatment of CAE. In this way, we can decide who needs to get treatment.
In this study, a total of 398 patients were consecutively selected among 4549 patients who underwent coronary angiography for suspected CAD in the catheterization laboratory of Antalya Education and Research Hospital from September 2014 through February 2015 (for six months) and from September 2015 through February 2016 (for six months), so the study was continued for a total of 12 months in two parts. The first group consisted of 201 patients (CAE group, 121 males; mean age, 61.2±6.4 years) with isolated CAE without significant stenosis. The second group consisted of 197 consecutive participants with coronary arteries angiographically shown to be ‘normal’ and without CAE (control group, 119 males; mean age, 62.4±5.8 years). Those excluded from this study included patients with any lesions in their coronary angiograms, along with patients with diabetes mellitus (DM), HT, hyperlipidemia (HL), pulmonary diseases (chronic obstructive pulmonary disease, pneumonia, a history of pulmonary emboli, etc.), chronic renal failure, chronic liver disorders, moderate severe valvular disease, a left ventricular systolic dysfunction on echocardiography (ejection fraction <50%), anemia, pregnancy, obstructive sleep apnea, hematological disorders, a known malignancy, electrolyte imbalance (an abnormal value of potassium, calcium, sodium, or magnesium), a previous gastrectomy, an intestinal malabsorption syndrome, increased high-sensitive C-reactive protein (hs-CRP) level or any inflammatory process, and also drug history (including anti-gout agents, anti-inflammatory agents, calcium, vitamin D, and anti-depressive agents). Inflammatory processes were identified as those that increased hs-CRP, TNF α, IL 6, fibrinogen, white blood cells, or the neutrophil-to-lymphocyte ratio (NLR); also inclusive of any chronic disease such as DM, HT, and CAD for the purposes of this study. Since the level of vitamin D differs seasonally due to exposure to sunlight, this study was started in the winter season and was continued up to the end of February. This study complied with the declaration of Helsinki and was approved by the local ethics committee.
Blood samples were drawn from an antecubital vein before coronary angiography after a 12-hour overnight fast and analysed spectrophotometrically on an (auto) Architect C16000 Clinical Chemistry Analyzer (Abbott Inc., Abbott Park, IL, USA) using an enzymatic-colorimetric assay. Fasting blood glucose, blood pressure, hemoglobin A1c (HbA1c), creatinine, fibrinogen, thyroid stimulating hormone (TSH), parathyroid hormone (PTH), hs-CRP, total cholesterol, low-density lipoprotein cholesterol, high-density cholesterol, and triglyceride levels were recorded. 25-hydroxy vitamin D levels were measured using BioSource's 25OH-Vit D3-Ria-CT Kit (BioSource Europe S.A. Rue de L'Industrie, 8, B-1400 Nivelles, Belgium).
For whole blood count (hematocrit, hemoglobin, leukocytes, and platelets), the blood samples were collected in tubes with ethylenediaminetetraacetic acid and analysed on a Coulter LH 780 Hematology Analyzer (Beckman Coulter Ireland Inc., Mervue, Galway, Ireland) device using the impedance and optic scatter method. The body mass index (BMI) was calculated by dividing weight in kg by the square of height in meters (kg/m2).
Coronary angiograms were performed with a femoral approach using the Judkins technique, without the use of nitroglycerin, using the Siemens Axiom Artis DFC (Siemens Medical Solutions, Erlangen, Germany). Coronary angiograms were analysed by two blinded interventional cardiologists without the knowledge of the clinical status and laboratory measurements of the participants. According to the results of coronary angiography, significant coronary artery stenosis was defined as ≥50% of major coronary arteries; also, CAE was defined as the segmental or diffuse dilatation of the coronary arteries to a >1.5-fold diameter of the adjacent segments of the same artery or of different arteries, as following Falsetti and Carroll.7)
Transthoracic echocardiography was performed on individuals before they were discharged-using the Philips EPIQ 7 model 3D-featured echocardiography device (Philips Healthcare 3000 Minuteman Road Andover, MA, USA) and 2.5 MHz probein Antalya Education and Research Hospital Cardiology Clinic. LVEF was calculated with a modified Simpson method using end-diastole and systole volumes measured by echocardiography.
Data acquired from the study was recorded in SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Categorical measurements were recorded as numbers and percentages, while continuous measurements were recorded as averages with standard deviations. The correspondence of continuous variables with normal distributions was analysed with the Shapiro Wilk test. Accordingly, Student's T test and Mann-Whitney U test were used for determining differences between groups according to the presence of ectasia of these variables. We measured sensitivity and specificity values under a receiver operating characteristic (ROC) curve to find out whether vitamin D measurements were effective in the presence of ectasia.
Univariate and multivariate regression analyses were done to estimate (with a confidence interval [CI] of 95%) the presence of ectasia by considering age, smoking, white blood cell, fibrinogen, hs-CRP, TNF α, IL 6, NLR, systolic blood pressure, diastolic blood pressure, HbA1C, fasting blood glucose, and vitamin D levels as study parameters. Box and Whisker Plot graphing was drawn with MedCalc® v15.4 packaged software. A value of p<0.05 was accepted as being statistically significant.
The baseline characteristics of the study groups are shown in Table 1. According to these data, the prevalence of cardiovascular risk factors such as age, gender, smoking, and lipid panels did not differ among the groups (for all, p>0.05). Also, these groups were similar for BMI, HbA1c, fasting glucose panel, blood pressure, LVEF, Hb, creatinine, and TSH. Most importantly, inflammatory markers such as hs-CRP, TNF α, IL 6, fibrinogen, white blood cell, and NLR did not differ among these groups. The average PTH level of patients was higher than the average PTH level of controls (41.8±15.1 pg/mL vs. 19.1±5.81 pg/mL; p<0.001). Also, the average 25-OH vitamin D level of patients was lower than the average 25-OH vitamin D level of controls (14.5±6.3 ng/mL vs. 24.6±9.3 ng/mL; p<0.001) (Table 1).
Variables found to be statistically significant in univariate analyses were entered into multivariate logistic regression analysis. Vitamin D (odds ratio [OR]=0.818, 95% CI 0.789-0.901, p<0.0001) was independent correlates of the presence of isolated CAE (Table 2).
ROC analysis was performed to find out the cut-off value for vitamin D (Fig. 1). In ROC curve analysis, the cut-off value for vitamin D between the control group and patients was 10.8 and 85.6% sensitivity and 75.2% specificity (AUC: 0.854, 95% CI: 0.678-0.863) were observed. Furthermore, we analysed vitamin D according to statistics values has been shown on the Box and Whisker Plot for all groups (Fig. 2).
In this study, we investigated the importance of vitamin D in patients with CAE without any inflammatory processes. This selection should be emphasized, because many chronic diseases fall into this condition and it may affect the role of this marker which projected into our hypothesis.
As known, the pathophysiology of CAE has not yet been fully disclosed, although multiple abnormalities including inflammation, neurohormonal process, vasculitis, and atherosclerosis have been reported.5)10)11)12)13)14) It might be a variant of the CAD. This disease is commonly asymptomatic; on the other hand, CAE can cause angina pectoris and even MI with vasospasm, dissection, or thrombus in patients without CAD.15) CAE occurs 4 times more frequently in males than in females and in individuals who have risk factors for CAD, such as smokers and diabetics.16) The disease can cause the heart tissue to be deprived of blood and die due to decreased blood flow, and can cause blockages due to blood clots or spasms of the blood vessel.15) CAE also weakens the coronary arteries' walls; thus, they can rupture and result in death. The damage can result in angina (chest pain) and is a common complaint among these patients.15)16) It has been discovered that the disease normally occurs most often in the right coronary artery, followed by the left anterior descending artery, and finally, the left anterior circumflex artery.16)
As previously mentioned, CAE is an inflammatory process,12)13)14)17) and there is a study that proves it to be so. In a study conducted by Yalcin et al.,17) there is a significant statistical result between NLR and CAE, and it was concluded that we can also evaluate these results by using Markis classification.18) Also, in another study, redcell-distribution (RDW) was used as an easily-accessible marker: Isik et al.19) and Guo et al.20) proposed some possible mechanistic explanations of the relationship between RDW and CAE, and found that elevation in the RDW values is associated with both the presence and severity of the CAE. Furthermore, many markers like homocysteine, folic acid, platelet activity, adhesion molecules, and serum apelin levels21)22)23)24) can predict CAE and have, in the majority, been reported to do so with statistical significance.
In our study, we chose vitamin D to explain the pathophysiology of CAE, because the effects of vitamin D on the coronary artery have not yet been fully disclosed. Vitamin D is a subject of study in many areas of medicine, such as heart disease, endocrine disorders, neurological diseases, and muscle diseases.25)26)27) In humans, the most important compounds are vitamin D3 (also known as cholecalciferol) and vitamin D2 (ergocalciferol).28) Cholecalciferol and ergocalciferol can be taken from the diet and from supplements. Also dermal synthesis of vitamin D from cholesterol is dependent on sun exposure. Vitamin D from the diet or from sunlight-mediated dermal synthesis is biologically inactive; activation requires enzymatic conversion in the liver and kidney,25) so it is necessary to fulfil its function properly in these organs.
In many studies, vitamin D (like CAE) has been pointed to with regards to the relationship with the inflammation processes. Many of the studies have emphasized that vitamin D reduces oxidative damage.22)24) In the study which is similar to ours, the relationship between vitamin D and CAE has been emphasized; but in the study population, inflammation processes could not be ruled out.9) In our study, we have also used NLR. We have seen, in many studies of markers other than the traditional markers, an exclusion of inflammation processes.
As has been known, vitamin D deficiency has been associated with augmented RAAS activity4) and then RAAS activation in creating a thinning of the vessel wall, so this reveals aneurysmal formations. However, some studies have shown that impact of RAAS is often associated with inflammation.4) So basically, there is a need to search for a foundation of pathophysiology other than inflammation. Therefore, our study design is comparatively more suitable for such a marker. In our study, inflammatory chronic diseases such as HT, DM, HL and CAD were excluded so that hs-CRP, TNF α, IL 6, white blood cells, and NLR intended to show inflammatory activity was not statistically different. Moreover, we have not seen any signs of inflammation-which was a confounding factor for the role of RAAS or vitamin D CAE pathophysiology.
On the other hand, the study by Koli and Keski-Oja29) made the connection that a relationship exists between vitamin D and serum proteolytic enzymes. Vitamin D and its derivatives are potent regulators of these enzymes. As is known, aneurysmati segments demonstrate a marked degradation of the medial collagen and elastin fibers-with disruption of the internal and external elastic lamina. These findings, in association with the observation that cases in which the media was intact and uninvolved had no evidence of ectasia, suggest that the enzymatic degradation of the media may be a key component in the pathogenesis of coronary ectasia.8) So, classes of proteolytic enzymes such as cystein proteinases and serine proteinases may play an important role in the pathogenesis of coronary ectasia.30)
In our study, the vitamin D levels of the CAE patients were significantly lower than those of the control group. And the iPTH levels of CAE patients were higher than those of the control group. This finding showed that vitamin D might have a pathophysiological mechanism which acts independently of the inflammatory process's role in developing coronary ectasia or possibly other vascular aneurysms. Further studies should be done to describe these mechanisms (Fig. 3).
We found that there is an association between vitamin D and CAE in patients who had no inflammatory processes. Our study may provide evidence for the role of vitamin D as being a non-inflammatory factor in the pathophysiology of CAE. This may provide additional evidence for the role of vitamin D in CAD.
Figures and Tables
Fig. 1
ROC analysis and cut-off value for Vitamin D. ROC: receiver operating characteristic, AUC: area under the curve. CI: confidence interval.

Fig. 3
Diagram of factors affecting ectasia and possible Vitamin D mechanism. RAAS: renin angiotensin aldosterone system.
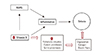
Table 1
Comparison of basic clinical and biochemical features of patients and controls

Values are presented as mean±standard deviation or number (%). BMI: body mass index, TSH: thyroid stimulating hormone, PTH: parathyroid hormone, hs-CRP: high sensitive C-reactive protein, TNF α: tumor necrosis factor α, IL 6: interleukin 6, Hb: hemoglobin, WBC: white blood cell, LDL-C: low density lipoprotein cholesterol, NEU/LYM: neutrophil/lymphocyte, SBP: systolic blood pressure, DBP: diastolic blood pressure, LVEF: left ventricular ejection fraction (normal range of hs-CRP: <1.0 mg/L)
Table 2
Independent predictors for the presence of ectasia in univariate and multivariate logistic regression analysis

References
1. Giovannucci E. What is the optimal vitamin D level for health? Therapy. 2008; 5:655–658.
2. Izadpanah M, Khalili H. Potential benefits of vitamin D supplementation in critically ill patients. Immunotherapy. 2013; 5:843–853.
3. Zittermann A, Pilz S, Börgermann J, Gummert JF. Calcium supplementation and vitamin D: a trigger for adverse cardiovascular events? Future Cardiol. 2011; 7:725–727.
4. Kota SK, Kota SK, Jammula S, et al. Renin-angiotensin system activity in vitamin D deficient, obese individuals with hypertension: an urban Indian study. Indian J Endocrinol Metab. 2011; 15:Suppl 4. S395–S401.
5. Sata M, Fukuda D. Chronic inflammation and atherosclerosis: a critical role for renin angiotensin system that is activated by lifestyle-related diseases. Inflamm Regen. 2011; 31:245–255.
6. Brady AR, Fowkes FG, Thompson SG, Powell JT. Aortic aneurysm diameter and risk of cardiovascular mortality. Arterioscler Thromb Vasc Biol. 2001; 21:1203–1207.
7. Falsetti HL, Carrol RJ. Coronary artery aneurysm. A review of the literature with a report of 11 new cases. Chest. 1976; 69:630–636.
8. Markis JE, Joffe CD, Cohn PF, et al. Clinical significance of coronary arterial ectasia. Am J Cardiol. 1976; 37:217–222.
9. Demir M, Demir C, Keçeoğlu S. The relationship between vitamin D deficiency and coronary artery ectasia. Postepy Kardiol Interwencyjnej. 2014; 10:238–241.
10. Koc F, Kalay N, Ardic I, et al. Antioxidant status and levels of antioxidant vitamins in coronary artery ectasia. Coron Artery Dis. 2011; 22:306–310.
11. Lin CT, Chen CW, Lin TK, et al. Coronary artery ectasia. Tzu Chi Med J. 2008; 20:270–274.
12. Dogan A, Tuzun N, Turker Y, Akcay S, Kaya S, Ozaydin M. Matrix metalloproteinases and inflammatory markers in coronary artery ectasia: their relationship to severity of coronary artery ectasia. Coron Artery Dis. 2008; 19:559–563.
13. Finkelstein A, Michowitz Y, Abashidze A, Miller H, Keren G, George J. Temporal association between circulating proteolytic, inflammatory and neurohormonal markers in patients with coronary ectasia. Atherosclerosis. 2005; 179:353–359.
14. Kocaman SA, Taçoy G, Sahinarslan A, Cengel A. Relationship between total and differential leukocyte counts and isolated coronary artery ectasia. Coron Artery Dis. 2008; 19:307–310.
15. Krüger D, Stierle U, Herrmann G, Simon R, Sheikhzadeh A. Exercise-induced myocardial ischemia in isolated coronary artery ectasias and aneurysms (“dilated coronopathy”). J Am Coll Cardiol. 1999; 34:1461–1470.
16. Hartnell GG, Parnell BM, Pridie RB. Coronary artery ectasia. Its prevalence and clinical significance in 4993 patients. Br Heart J. 1985; 54:392–395.
17. Yalcin AA, Topuz M, Akturk IF, et al. Is there a correlation between coronary artery ectasia and neutrophil-lymphocyte ratio? Clin Appl Thromb Hemost. 2015; 21:229–234.
18. Markis JE, Joffe CD, Cohn PF, Feen DJ, Herman MV, Gorlin R. Clinical significance of coronary arterial ectasia. Am J Cardiol. 1976; 37:217–222.
19. Isik T, Kurt M, Ayhan E, et al. Relation of red cell distribution width with presence and severity of coronary artery ectasia. Clin Appl Thromb Hemost. 2012; 18:441–447.
20. Guo YL, Luo SH, Tang Y, Li JJ. Association of red cell distribution width with the presence of coronary artery ectasia. Clin Lab. 2014; 60:199–205.
21. Bilik MZ, Kaplan İ, Yıldız A, et al. Apelin levels in isolated coronary artery ectasia. Korean Circ J. 2015; 45:386–390.
22. Turhan H, Erbay AR, Yasar AS, et al. Plasma soluble adhesion molecules; intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and E-selectin levels in patients with isolated coronary artery ectasia. Coron Artery Dis. 2005; 16:45–50.
23. Koc F, Ardic I, Erdem S, et al. Relationship between L arginine/asymmetric dimethylarginine, homocysteine, folic acid, vitamin B levels, and coronary artery ectasia. Coron Artery Dis. 2010; 21:445–449.
24. Yasar AS, Erbay AR, Ayaz S, et al. Increased platelet activity in patients with isolated coronary artery ectasia. Coron Artery Dis. 2007; 18:451–454.
25. Ke CY, Yang FL, Wu WT, et al. Vitamin D3 reduces tissue damage and oxidative stress caused by exhaustive exercise. Int J Med Sci. 2016; 13:147–153.
26. Michos ED. Vitamin D deficiency and the risk of incident Type 2 diabetes. Future Cardiol. 2009; 5:15–18.
27. Tohari AM, Zhou X, Shu X. Protection against oxidative stress by vitamin D in cone cells. Cell Biochem Funct. 2016; 34:82–94.
28. Holick MF, Schnoes HK, DeLuca HF, Suda T, Cousins RJ. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry. 1971; 10:2799–2804.
29. Koli K, Keski-Oja J. 1alpha, 25-dihydroxyvitamin D3 and its analogues down-regulate cell invasion-associated proteases in cultured malignant cells. Cell Growth Differ. 2000; 11:221–229.
30. Liu J, Sukhova GK, Yang JT, et al. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis. 2006; 184:302–311.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download