Abstract
Background and Objectives
Thoracic endovascular aortic repair exhibits limitations in cases where the aortic pathology involves the aortic arch. We had already developed a fenestrated aortic stent graft (FASG) with a preloaded catheter for aortic pathology involving the aortic arch. FASG was suitable for elective cases.
Materials and Methods
An aortic arch stent graft with a window-shaped fenestration (FASG-W) for supra-aortic arch vessels is suitable for emergent cases. This study aims to test a FASG-W for supra-aortic arch vessels and to perform a preclinical study in swine to evaluate the safety and efficacy of this device. Six FASG-Ws with 1 preloaded catheter were advanced through the iliac artery in 6 swine. The presence of endoleak and the patency and deformity of the grafts were examined with computed tomography (CT) at 4 weeks postoperatively. A postmortem examination was performed at 8 weeks. The mean procedure time for FASG-W was 27.15±4.02 minutes. The mean time for the selection of the right carotid artery was 5.72±0.72 minutes.
Thoracic endovascular aortic repair (TEVAR) is an effective therapy for various thoracic aortic pathologies, including aortic aneurysms, aortic dissections, and traumatic aortic transections, particularly in patients with a high operative risk.1)2)3)4)5) TEVAR has a short procedure time, decreased complication rate, and low hospital mortality compared to open surgery to repair the thoracic aorta.5)6)7)8)9) However, it is difficult to perform TEVAR in patients with a short proximal stent graft landing zone.10)11) Most companies that fabricate thoracic aortic stent grafts recommend their use in patients with a proximal landing zone >15 mm. When patients have a short proximal landing zone near the aortic arch, there is increased risk of endoleak, migration of the stent graft due to angulation of the aortic arch, and high blood pressure in the aorta.6)9)12) Thirty to fifty percent of cases with aortic aneurysm, aortic dissection, or traumatic aortic transection involve the aortic arch.13)14)15)16) Several branched aortic stent grafts and fenestrated aortic stent grafts for aortic arch disease have been reported, but these products are not commercially available because of various issues.17)18)19)20) We had already developed a convenient fenestrated aortic arch stent graft (FASG) with a preloaded catheter for the supra-aortic arch vessel and had performed a preclinical study to evaluate this FASG in swine.21) Unfortunately, it takes more than 3 days to create the FASG for each case. For emergent cases, we developed an aortic arch stent graft with a window-shaped fenestration (FASG-W) for supra-aortic arch vessels. FASG-Ws are ready-made and are composed of various sized stent grafts for emergent cases. The study aims were to test an FASG-W for supra-aortic arch vessel and to perform a preclinical study in swine to evaluate the safety and efficacy of this device.
The FASG-W is composed of a main stent graft with a window-shaped fenestration and a preloaded catheter, and a stent graft for the supra-aortic arch vessel. The FASG-W developed here has a preloaded catheter (1.14 mm in diameter) inside the main delivery system to select the innominate and the right carotid artery. The preloaded catheter links to the proximal part of the window-shaped fenestration, and the distal port of the preloaded catheter protrudes from the distal portion of the deployment section of the main delivery system. The preloaded catheter links 10 mm from the proximal end of the window-shaped fenestration to easily select the innominate and right carotid artery. Pigs have only two carotid arteries arising from the aorta, with no innominate artery or left subclavian artery. A 0.035-inch guidewire can reach the right carotid artery through this preloaded catheter (Fig. 1). This window-shaped fenestration is indicated with a window-shaped gold marking, and the opposite side of the fenestration is indicated with a straight gold line for easy identification (Fig. 2). This window-shaped fenestration is 15 mm in width and 40 mm in length. The framework of the FASG-W is composed of 0.234-mm nitinol, while the graft is made from polytetrafluoroethylene (PTFE). The diameter and length of the FASG-W are 34 mm and 150 mm, respectively. The bare area of the FASG-W (i.e., the area lacking PTFE around the nitinol frame) is 30 mm in length from the proximal end of the graft and is oversized at a diameter of 38 mm to prevent the migration of the FASG-W. The proximal end of the FASG-W is tied up at 2 points that are movable when the FASG-W is partially deployed and can be untied at the end of the stent graft deployment (Fig. 2, 3). The profile of the FASG-W is 18 French, including the preloaded catheter. The stent graft for the supra-aortic arch vessel is 10 mm in diameter and 40 mm in length. The stent graft for the supra-aortic arch vessel was designed to have flare-shaped bare areas 5 mm in length at both the proximal and distal ends of the stent graft.
Six FASG-Ws with a preloaded catheter and a window-shaped fenestration were placed through the iliac artery in 6 swine (70-80 kg). The presence of endoleaks and the patency and deformity of the grafts were evaluated with computed tomography (CT) at 4 weeks after the procedure. Postmortem examination was performed at 8 weeks to evaluate the gross morphology, patency, and deformity of the FASG-Ws and stent grafts for the right carotid artery. This preclinical study protocol was approved by the animal experimental committee of Pusan National University Hospital.
The pigs were sedated with an intramuscular injection of ketamine hydrochloride (10 mg/kg) and xylazine (2 mg/kg). Sedation was maintained with inhaled isofluorane. The iliac artery was then exposed by a retroperitoneal approach, and 10000 units of heparin were administered intravenously. The diameter of the iliac artery was measured following angiography. We inserted an 18-French sheath into the iliac artery and placed a 0.035-inch extra-stiff guidewire (Lunderquist™, COOK, Bloomington, IN, USA) in the ascending aorta. A marked pigtail catheter was inserted through the other iliac artery into the aorta to examine both the aorta and the carotid arteries during the procedure. The marked window-shaped fenestration was identified in advance under fluoroscopy (Fig. 2). The FASG-W was advanced into the aortic arch, with the window-shaped fenestration facing the carotid artery. We partially deployed the FASG-W up to 1 cm proximal to the window-shaped fenestration and selected the right carotid artery using the 0.035-inch hydrophilic guidewire (Terumo, Tokyo, Japan). We moved the FASG-W forward to fit the proximal part of the window-shaped fenestration into the right carotid artery and completely deployed the FASG. The delivery sheath of the FASG was removed from the aorta, but the guidewire for the right carotid artery was maintained. We then advanced the stent graft (10 mm × 40 mm) for the right carotid artery. Aortography was performed to assess the flow of the carotid arteries to detect endoleaks (Fig. 3).
Six pigs were successfully implanted with FASG-Ws. The mean procedure time for the FASG-W was 27.2±4.0 minutes. Additionally, the mean time for the selection of the right carotid artery was 5.7±0.6 minutes (Table 1). Major adverse events such as death, cerebral ischemia, limb ischemia, organ infarction, paraplegia, serious infection, and vascular complications were observed in the 6 pigs. All 6 pigs survived the 8-week observational period. Vascular damage to the iliac artery, aorta, and carotid artery was not detected in any of the 6 pigs during the procedure (Table 1). Moreover, none of the pigs exhibited migration of the FASG-W. We were able to easily select the right carotid artery in all 6 pigs using the preloaded catheter and had no instances of selection failure for the right carotid artery. The FASG-W moved easily after partial deployment to fit the window-shaped fenestration into the carotid arteries.
For the FASG-W group (n=6), no endoleaks, no retrograde type A aortic dissection, no disconnection of the stent grafts, and no occlusion of the stent grafts for the right carotid artery were observed in the CT findings at 4 weeks (Fig. 4). The 6 pigs were sacrificed at 8 weeks for autopsy. No disconnection or tearing of the stent grafts, no fractures in the stent grafts, no retrograde type A aortic dissection, and no occlusion of the stent grafts for the right carotid artery were found in the postmortem gross findings (Fig. 5).
A short landing zone for aortic arch pathology can be problematic when performing TEVAR. In response to this particular issue, a few doctors have developed fenestrated or branched stent grafts for aortic arch disease. Inoue et al.17) developed a branched aortic stent graft for aortic arch disease and attempted to use it in the treatment of 15 patients with thoracic aortic aneurysms and aortic dissections. The Inoue et al.17) branched stent graft is not commercially available because the stent graft has a large profile of 22-24 French, a long procedure time, a complicated procedure method, and requirement of surgical exposure of the carotid and subclavian arteries. The free end of the traction wire attached to the tip of the branched stent graft was caught and pulled back by a gooseneck snare wire through the left subclavian, carotid, and innominate arteries to deploy the folded branch stent graft. This complicated procedure method resulted in a long procedure time. In our previous report, we developed one-branch and two-branch FASG with preloading catheters and reported a successful preclinical study result.21) Our FASG had a small size, short procedure time, and simple procedure method. Moreover, the FASG had a size of 18 French, which is comparable to the 7-mm-diameter of the iliac artery. Of note, no iliac artery perforation was observed in our previous study. The mean procedure time was very short because our FASG had a preloaded catheter with a diameter of 1.14 mm inside the main delivery system to select the carotid, innominate, and subclavian arteries. This system reduced the procedural time and simplified the procedure method.
Unfortunately, it takes more than 3 days to create the FASG for each patient. Patients have various sized aortas, innominate arteries, left carotid arteries, and left subclavian arteries. The distances among the supra-aortic arch vessels are individually varied. We can create an FASG after measuring the innominate artery, left carotid artery, left subclavian artery, and aorta of patients on CT. Therefore, we developed an FASG-W to protect the supra-aortic arch vessels in emergent patients with aortic arch pathology. FASG-Ws are ready-made and are composed of various-sized stent grafts for emergent cases. We had measured the diameters of the supra-aortic arch vessels and aortas in many patients with aortic arch pathology. Three different diameters of FASG-Ws with three different sized window fenestrations were prepared for emergent cases with aortic arch pathology (diameters of the stent graft: 40 mm, 44 mm, 48 mm; sizes of the window fenestrations: 15 mm × 60 mm, 15 mm × 80 mm, 15 mm × 100 mm). In this preclinical study for FASG-W, the procedure time was short, selection of the right carotid artery was easy, and procedure steps were simple. There was no occlusion of either carotid artery, and no migration of the main stent graft or stent graft for the right carotid artery. The stent graft for the right carotid artery was deployed to prevent migration of the main stent graft and occlusion of the carotid artery. However, identification of the innominate and right carotid arteries in the diseased aorta of humans will be difficult because of the angulation of the diseased aortic arch and the variable location of the innominate and carotid arteries. Therefore, we prepared 0.035 inch and 0.018 inch shapeable guidewires to allow for easier selection of the innominate and right carotid arteries. Sometimes, a gooseneck snare inserted through the right brachial artery can be used to catch the preloading wire in patients with an angulated aortic arch.
Pigs have two carotid arteries arising from the aorta, with no innominate artery or left subclavian artery. FASG-W has a preloaded catheter, and the 0.035-inch guidewire can be placed into the right carotid artery in advance. This wire can prevent the migration of the FASG-W during deployment and can accurately fit the branch stent graft to the right carotid artery. Furthermore, the delivery sheath of the FASG-W was removed from the aorta, with the guidewires for the right carotid artery in place, and the advanced branch stent graft was fitted to the right carotid artery. The stent graft for the right carotid artery was used to prevent migration of the FASG-W. We considered that a weak portion of the FASG-W might result in endoleak through the window-shaped fenestration. Therefore, we only recommend an FASG-W for emergent cases with aortic arch pathology located in the lesser curvature or lateral side of the aortic arch. If the aortic arch pathology is involved in the greater curvature side of the aortic arch with supra-aortic vessel involvement, we recommend the one- or two-branch FASG. One- or two-branch FASGs were developed for elective procedures. We can measure the diameters of the aorta, innominate artery, left carotid artery, and left SCA individually. It takes more than 3 days to create the FASG for each case. We can precisely insert the FASG and stent graft for the innomiate artery, carotid artery, and left subclavian artery. FASG-W is a pre-made stent graft developed for emergency cases. Various sized FASG-Ws can be designed in advance for various types of emergent patients (diameters of the stent graft: 40 mm, 44 mm, 48 mm; sizes of the window fenestration: 15 mm × 60 mm, 15 mm × 80 mm, 15 mm × 100 mm). It is difficult to precisely insert the FASG-W into the innominate, carotid, and left subclavian arteries, but the use of the FASG-W allows for the possibility of preserved blood flow for supra-aortic arch vessels. FASG-Ws are pre-made stent grafts that take into consideration the aorta size and the diameters of supra-aortic arch vessels. FASG-W usage may be more frequent with respect to type 3 endoleaks compared to one- or two-branch FASGs. As mentioned above, we do not recommend the use of FASG-Ws in cases with pathology in the greater curvature of the aorta. We only recommend FASG-Ws for pathology in the lesser curvature or lateral wall of the aorta. One- or two-branch FASGs and FASG-Ws can be used in combination.
The selection time for the carotid artery in FASG-W was slightly longer than that for the one-branch FASG (5.7 min vs. 4.8 min). We selected the right carotid artery for FASG-Ws and the left carotid artery for one-branch FASGs. The angulation from the aorta to the left carotid artery is straighter than that to the right carotid artery. The angulation required more time to select the right carotid artery in the FASG-W. The selection time of the carotid artery for FASG-W was shorter than that of the two-branch FASG (6.2 min).
No endoleaks were observed in the preclinical animal study. In the postmortem gross findings, the main body of the FASG-W and the stent graft for the right carotid artery tightly adhered to the aorta and right carotid artery.
Based on our findings, we submitted an application for a clinical study to the Korean Food and Drug Administration and will be performing a clinical study in the near future.
Our animal study has several study limitations. In our animal study, we were unable to create an aortic arch aneurysm model. To our knowledge, the development of an aortic arch aneurysm model is not possible. Therefore, it was difficult to precisely estimate the occurrence of endoleaks through the window-shaped fenestration. However, we did not detect any significant endoleaks in the preclinical animal study.
Humans are anatomically different from pigs in several respects relevant to this study. Although pigs possess two carotid arteries that are uniform in size and located at regular intervals, the innominate, carotid, and left subclavian arteries are anatomically variable in humans. Therefore, we had measured the diameters and intervals of supra-aortic arch vessels and aortas in many patients with aortic arch pathology. Three different diameters of FASGs with three different sized window fenestrations were prepared for emergent patients with aortic arch pathology. Moreover, the angulation of the aortic arch in pigs is different from that in humans. Therefore, we prepared 0.035 inch and 0.018 inch shapeable guidewires to enable easier selection of the innominate and right carotid arteries.
The FASG-W with the preloading catheter for the innominate and right carotid arteries was developed for emergent cases where the aortic pathology involves the aortic arch with a short proximal landing zone. In this study, we performed a preclinical study with this device in swine. The procedure with the FASG-W was able to be performed safely, had a short procedure time, and involved an easily performed technique. No endoleaks; no aortic dissections; and no disconnection, occlusion, or tearing of the stent grafts were found in the CT or postmortem gross findings. In conclusion, the FASG-Ws with preloaded catheters developed for this study were comparatively safe and convenient in this preclinical study with swine.
Figures and Tables
Fig. 1
The FASG-W has a preloaded catheter (1.14 mm in diameter) inside the main delivery system for selection of the right carotid artery (red arrow). The preloaded catheter links to a window-shaped fenestration (red arrow), and the distal port of the preloaded catheter protrudes from the distal portion of the deployment section of the main delivery system. A 0.035-inch guidewire can reach the right carotid artery through the preloaded catheter. The size of the FASG-W is 18 French, including the preloaded catheter. FASG-W: fenestrated aortic stent graft window-shaped fenestration.

Fig. 2
The framework of the FASG-W is composed of 0.234-mm nitinol. The graft of the FASG is made of polytetrafluoroethylene. The FASG-W is 34 mm and 150 mm in diameter and length, respectively. The bare area of the FASG-W is 30 mm in length from the proximal end of the graft, with an oversized diameter of 38 mm to prevent migration. (A) The proximal end of the FASG-W is tied up at 2 points to allow for movement when the FASG-W is deployed partially and is able to be untied at the end of stent graft deployment. (A-D) The fenestration is indicated with a window-shaped gold mark, and the opposite side of the fenestration is indicated with a straight gold mark for easy identification. FASG-W: fenestrated aortic stent graft window-shaped fenestration.
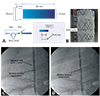
Fig. 3
The FASG-W was advanced into the aortic arch, and the window-shaped fenestration (A) faced the carotid artery. We then partially deployed the FASG-W to the proximal portion of the fenestration (B) and selected the right carotid artery with the 0.035-inch hydrophilic guidewires (C). We pushed the FASG-W up to fit the window-shaped fenestration into the carotid arteries (D) and completely deployed the FASG-W (E, F). The delivery sheath of the FASG was removed from the aorta, but the guidewire for the right carotid artery was maintained. We then advanced and deployed the stent graft for the right carotid artery (G, H). Aortography was conducted to examine the flow of the carotid arteries and to detect endoleaks (I). FASG-W: fenestrated aortic stent graft window-shaped fenestration, FASG: fenestrated aortic stent graft.
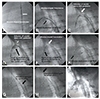
Fig. 4
CT findings at 4 weeks. (A, B) The blood flow of the carotid arteries was preserved through the window-shaped fenestration. (C) Stent graft for the right carotid artery was connected to the aorta and was patent. (D-F) The main stent graft of FASG-W was in the aorta. There was no endoleak, disconnection, or dissection. CT: computed tomography, FASG-W: fenestrated aortic stent graft window-shaped fenestration.
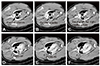
Fig. 5
Postmortem gross findings. (A-D) There was no disconnection or tearing of the stent graft, no fractures in the stent graft, and no occlusion of the stent graft for the right carotid artery. (C, D) The blood of both carotid arteries was preserved through the window-shaped fenestration.
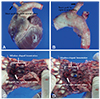
Table 1
Procedure-related findings with the FASG-W (n=6)

References
1. Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999; 340:1546–1552.
2. Dake MD, Miller DC, Semba CP, Mitchell RS, Walker PJ, Liddell RP. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med. 1994; 331:1729–1734.
3. Greenberg R, Resch T, Nyman U, et al. Endovascular repair of descending thoracic aortic aneurysms: an early experience with intermediate-term follow-up. J Vasc Surg. 2000; 31(1 Pt 1):147–156.
4. Nienaber CA, Rousseau H, Eggebrecht H, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009; 120:2519–2528.
5. Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J. 2012; 33:26–35b.
6. Shah AA, Barfield ME, Andersen ND, et al. Results of thoracic endovascular aortic repair 6 years after United States Food and Drug Administration approval. Ann Thorac Surg. 2012; 94:1394–1399.
7. Zhang H, Wang ZW, Zhou Z, Hu XP, Wu HB, Guo Y. Endovascular stent-graft placement or open surgery for the treatment of acute type B aortic dissection: a meta-analysis. Ann Vasc Surg. 2012; 26:454–461.
8. Narayan P, Wong A, Davies I, et al. Thoracic endovascular repair versus open surgical repair - which is the more cost-effective intervention for descending thoracic aortic pathologies? Eur J Cardiothorac Surg. 2011; 40:869–874.
9. Makaroun MS, Dillavou ED, Wheatley GH, Cambria RP. Gore TAG Investigators. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J Vasc Surg. 2008; 47:912–918.
10. Lee SH, Chung CH, Jung SH, et al. Midterm outcomes of open surgical repair compared with thoracic endovascular repair for isolated descending thoracic aortic disease. Korean J Radiol. 2012; 13:476–482.
11. Al-Nouri O, Moeller C, Borrowdale R, Milner R. Late complication of thoracic endovascular stent-grafting. Ann Vasc Surg. 2011; 25:982.e1–982.e4.
12. Lee CJ, Rodriguez HE, Kibbe MR, Malaisrie SC, Eskandari MK. Secondary interventions after elective thoracic endovascular aortic repair for degenerative aneurysms. J Vasc Surg. 2013; 57:1269–1274.
13. Ullery BW, McGarvey M, Cheung AT, et al. Vascular distribution of stroke and its relationship to perioperative mortality and neurologic outcome after thoracic endovascular aortic repair. J Vasc Surg. 2012; 56:1510–1517.
14. Grabenwöger M, Alfonso F, Bachet J, et al. European Association for Cardio-Thoracic Surgery (EACTS). European Society of Cardiology (ESC). European Association of Percutaneous Cardiovascular Interventions (EAPCI). Thoracic Endovascular Aortic Repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2012; 42:17–24.
15. Lee KN, Lee HC, Park JS, et al. The modified chimney technique with a thoracic aortic stent graft to preserve the blood flow of the left common carotid artery for treating descending thoracic aortic aneurysm and dissection. Korean Circ J. 2012; 42:360–365.
16. Sun Z, Mwipatayi BP, Allen YB, Hartley DE, Lawrence-Brown MM. Multislice CT angiography of fenestrated endovascular stent grafting for treating abdominal aortic aneurysms: a pictorial review of the 2D/3D visualizations. Korean J Radiol. 2009; 10:285–293.
17. Inoue K, Hosokawa H, Iwase H, et al. Aortic arch reconstruction by transluminally placed endovascular branched stent graft. Circulation. 1999; 100:19 Suppl. II316–II321.
18. Schneider DB, Curry TK, Reilly LM, Kang JW, Messina LM, Chuter TA. Branched endovascular repair of aortic arch aneurysm with a modular stent-graft system. J Vasc Surg. 2003; 38:855.
19. Ahanchi SS, Almaroof B, Stout CL, Panneton JM. In situ laser fenestration for revascularization of the left subclavian artery during emergent thoracic endovascular aortic repair. J Endovasc Ther. 2012; 19:226–230.
20. Wei G, Xin J, Yang D, et al. A new modular stent graft to reconstruct aortic arch. Eur J Vasc Endovasc Surg. 2009; 37:560–565.
21. Kim SP, Lee HC, Park TS, et al. Safety and efficacy of a novel, fenestrated aortic arch stent graft with a preloaded catheter for supraaortic arch vessels: an experimental study in Swine. J Korean Med Sci. 2015; 30:426–434.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download