Abstract
Management of severely dilated pulmonary artery (PA) associated with severe pulmonary hypertension from congenital heart disease remains controversial, primarily due to its rare nature and concern for perioperative unpredictable complications. Herein, we report a 25 year-old female with a severely dilated PA (up to 73 mm), who was successfully treated by a PA graft replacement by creating a Y-shaped conduit using a 28 mm hemashield tube in the main PA and a 20 mm hemashield tube in both proximal parts of the branch PA.
Pulmonary artery (PA) dilatation associated with severe pulmonary hypertension is a rare condition. Despite its rarity however, unexpected deaths may result more frequently than commonly believed.1)2) Possible mechanisms of sudden death include left main coronary artery (LMCA) compression by a markedly dilated PA and PA rupture or dissection with cardiac tamponade.3) There has been no standard treatment guideline for a dilated PA from severe pulmonary hypertension, as it is not only quite rare, but also associated with a challenging surgical treatment with concerns for perioperative unpredictable complications. Herein, we report a 25-year-old female with a severely dilated PA (up to 73 mm) and severe pulmonary hypertension from complete atrioventricular septal defect (AVSD), who was successfully treated by a PA graft replacement using a Y-shaped conduit creation with a 28 mm hemashield tube in the main pulmonary artery (MPA) and a 20 mm hemashield tube in both proximal parts of the branch PA.
A 20 year-old female patient was referred for further treatment of complete AVSD with a PA aneurysm associated with severe pulmonary hypertension. She was diagnosed as having a left isomerism with inferior vena cava (IVC) interruption, dextrocardia, complete AVSD with single atrium, ventricular septal defect, and bilateral superior vena cava (SVC). She was diagnosed at another hospital at 10 years old due to symptoms of dyspnea on exertion. Because she showed severe pulmonary hypertension at that time, she was diagnosed as being in an inoperable state at another hospital. After discharge from the hospital, she did not visit any hospital for 10 years. After entrance into the University at the age of 20, she visited another hospital to check her medical status. She was diagnosed again, was not to be operated on, and prescribed with oral furosemide and digoxin. She visited our hospital again to get a secondary opinion. When she visited our hospital, she complained of exercise intolerance (difficult to climb 2 floor stairs, NYHA class III). On examination, she showed a palpable systolic impulse at the second left intercostal space with a grade of 3/6 systolic murmur and 2/6 diastolic murmur at the lower and upper left sternal border, respectively.
A chest radiograph showed cardiomegaly and a dilated main pulmonary trunk and proximal branch PA (Fig. 1A). An echocardiogram revealed that an inlet VSD was closed by an aneurysm and there was another 5 mm apical muscular VSD. From the cardiac computed tomography (CT), the MPA was measured to be 73 mm on the axial plane at the level of aorticopulmonary window (Fig. 2A, B). A cardiac catheterization showed a mean PA pressure of 64 mmHg, a pulmonary vascular resistance (PVR) of 13.9 wood units at room air. The systemic arterial oxygen saturation (SaO2) was measured to be 83% at room air. After oxygen (10 L/min) was supplied, the mean PA pressure was 60 mmHg, PVR was 8.3 Wood units and SaO2 was measured to be 94% (Table 1). For the concern of irreversible hypertensive change in the PA, the option to operate was conceded and bosentan was started for 7 months to see the progression of PVR. After 7 months of bosentan treatment, cardiac catheterization revealed that the mean PA pressure dropped to 49 mmHg, and concomitantly PVR decreased to 10.2 Wood units and SaO2 was measured to be 87.2% at room air. After oxygen (10 L/min) was supplied, the mean PA pressure was 47 mmHg, PVR was 6.0 Wood units and SaO2 was measured to be 96.8%. We continued to give bosentan for the palliative medication. After 3 years of bosentan treatment, she showed maximal oxygen consumption (VO2 max) of 20.4 mL/kg/min and desaturated as 62% by pulse oximetry at peak exercise from the cardiopulmonary exercise test (Table 1).
Despite medical treatment for 5 years, the patient complained of orthopnea and worsening dyspnea (NYHA class III), accompanied by a further dilated PA (up to 80 mm) by echocardiography. Because we had concerns about PA dissection and intravascular thrombosis of PA, we finally decided to operate, performing reduction of MPA and ASD closure with fenestration. The operation was carried out via a median sternotomy with the use of a cardiopulmonary bypass. We replaced the MPA and proximal branch PA with a Y-shaped graft by a creation neo-MPA trunk using a 28 mm hemashield vascular graft and neo-branch proximal PA using a 20 mm hemashield vascular graft. She also underwent concomitant open-heart surgery including an atrial patch partitioning with an 8 mm fenestration, repair of the mitral valve cleft, division of right-sided SVC and anastomosis to the right atrial auricle with 16 mm polytetrafluoroethylene graft interposition to increase oxygen saturation and division of the remnant bilateral ductal artery (Fig. 3). The cardiopulmonary bypass time was 445 minutes and aortic clamping time was 110 minutes. Pathology of the resected PA specimen revealed a pulmonary aneurysm with myxoid degeneration of vascular wall (Fig. 4). However, postoperative course was not smooth.
Due to persistent bleeding after the operation, the patient underwent a hematoma evacuation and bleeding control twice. Bleeding focus was at an anastomosis site between the native right PA and graft mainly due to size discrepancy. Because of an acute kidney injury associated with massive bleeding and vancomycin-induced nephropathy, renal replacement therapy was required using hemodialysis between 5 days and 37 days and peritoneal dialysis by the 80th postoperative day. The patient was weaned from a mechanical ventilator on the 26th postoperative day and was discharged on the 89th postoperative day with bosentan, sidenafil citrate, thiazide, spironolactone, and aspirin.
After discharge, she showed increased VO2max of 23.9 mL/kg/min and desaturated only down to 80% at peak exercise at 1 year after the operation. Follow-up cardiac catheterization was performed 14 months after operation; the mean PA pressure was dropped to 49 mmHg and PVR decreased to 6.4 Wood units and SaO2 was measured to be 94% at room air (Table 1). The postoperative chest radiograph and cardiac CT revealed a decreased size of the neo-MPA and proximal branch PA with the remaining aneurysm of the lobar PA (Fig. 1B and 2C, D). She showed improvement of daily performance (could climb up to 12 floor stairs, NYHA class II) at her last visit, 22 months after postoperation.
Herein, we reported a 25 year-old female with a severely dilated PA (up to 80 mm by echocardiography) successfully treated by a PA graft replacement thtough a creation of a Y-shaped conduit using a 28 mm hemashield tube in an MPA and 20 mm hemashield tube in both proximal parts of the branch PA. Although the postoperative course was not easy, the patient's daily performance and quality of life definitely improved.
More than half of PA aneurysm cases are associated with congenital heart disease, where the pulmonary circulation may have significant volume and pressure overload, most frequently with patent ductus arteriosus. Some authors described that progressive dilatation of PA is related to changes on the vessel wall, rather than hemodynamic changes such as PA pressure, especially in low-pressure giant PA aneurysms including connective tissue disorders.4) According to Laplace's law, the larger the vessel radius, the larger the wall tension, regardless of PA pressure, any aneurysm would continue to enlarge, thinning the arterial wall, and subsequently rupture. PA aneurysm was defined as a pathologic dilatation of the PA to a diameter of at least 1.5-fold the normal diameter. One study proposed a cut-off of 48 mm in diameter of the PA aneurysm, which represented the risk of unexpected death.5) Another study proposed the risk factor for a dissection or rupture as an absolute PA diameter and growth rate (>75 mm and growth rate >2 mm/year).6)
Various complications of PA aneurysms such as airway compression, thrombus formation, LMCA compression and PA dissection may lead to unexpected mortality in one third of the patient populations.7)8) PA dissection occurred in 20%, more and is often associated with high intravascular pressure and longer duration of elevated PA pressure.6) Chest pain in pulmonary hypertension may often be a consequence of worsening pulmonary hypertension, right ventricle hypertrophy and strain leading to demand ischemia. Chest pain is also caused by LMCA extrinsic compression due to a dilated PA. The occurrence of LMCA compression correlates with increased PA pressure and PA diameter.1)8) Associated problems include exercise related chest pain, ventricular dysfunction, malignant arrhythmias due to myocardial ischemia and sudden death.5) In one study, risk factors predisposing to LMCA compression in pulmonary hypertension were younger age, severe PA dilatation (>40 mm), and a PA trunk/aorta ratio >1.2 (normal: 1.0). The treatment of LMCA extrinsic compression is also known to be safe by percutaneous coronary intervention.1)
Intravascular thrombi could be formed within aneurysm secondary to stasis from swirled blood flow, and may break off and lodge distally, which can lead to fatal pulmonary embolism. So, it has been generally accepted that anticoagulant treatment is necessary to PA aneurysm, although it may be suspended in cases of hemoptysis, progressive PA growth and PA dissection.
Williams and colleagues described the first report of repair of PA aneurysms in 1971.9) Thereafter, various treatments have been reported, including aneurysmorraphy and angioplasty. Graft replacement would obtain a better outcome reducing recurrent aneurysmal dilation although there is a risk of bleeding at the anastomosis site, like in our case.
In conclusion, we must pay special attention to such symptoms for patients with severe PA aneurysms. Moreover, surgical treatments could be considered as a palliative treatment for some patients with a severely dilated PA with pulmonary hypertension from a congenital heart anomaly. Palliative surgery could improve exercise capacity and delay the progression of the disease, in parallel with conventional pharmacologic treatments, especially for patients with a high risk of serious complications such as PA ruptures.
Figures and Tables
Fig. 1
Chest radiography. (A) Preoperative radiograph shows cardiomegaly and dilated main pulmonary trunk and its branches in central and peripheral localizations. (B) Postoperative radiograph (18 months after the operation) shows a reduction in the size of the main pulmonary trunk and arteries.
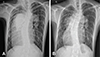
Fig. 2
Cardiac CT with 3D reconstruction. (A, B) Preoperative image shows severely dilated main pulmonary artery (measuring 73 mm) and its branches at the level of aorticopulmonary window. (C, D) Postoperative image (19 months after the operation) revealed a decreased size of neo-main pulmonary artery (measuring 28 mm) and proximal branch pulmonary arteries with remained aneurysm of lobar pulmonary arteries. CT: computed tomography.
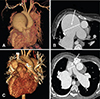
Fig. 3
Operative findings and procedures. (A) Postoperative diagnosis was a left isomerism with inferior vena cava interruption, dextrocardia, single atrium, complete atrioventricular septal defect with another muscular ventricular septal defect, bilateral superior vena cava, and pulmonary artery aneurysm with severe pulmonary hypertension. (B) Y-shaped graft replacement by a creation of 28 mm main pulmonary artery trunk using 20 mm hemashield. (C) Mitral valve cleft closure and atrial patch partitioning with 8 mm fenestration. (D) Right-sided superior vena cava division and anastomosis to right atrium auricle with 16 mm polytetrafluoroethylene graft interposition. MV: mitral valve, CS: coronary sinus.

Fig. 4
Pulmonary artery specimen. Resected pulmonary artery revealed huge pulmonary aneurysm with myxoid degeneration of the vascular wall.
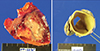
Table 1
Cardiac catheterization and cardiopulmonary exercise test prior to and after cardiac surgery

References
1. Mesquita SM, Castro CR, Ikari NM, Oliveira SA, Lopes AA. Likelihood of left main coronary artery compression based on pulmonary trunk diameter in patients with pulmonary hypertension. Am J Med. 2004; 116:369–374.
2. Degano B, Prevot G, Têtu L, Sitbon O, Simonneau G, Humbert M. Fatal dissection of the pulmonary artery in pulmonary arterial hypertension. Eur Respir Rev. 2009; 18:181–185.
3. Żyłkowska J, Kurzyna M, Florczyk M, et al. Pulmonary artery dilatation correlates with the risk of unexpected death in chronic arterial or thromboembolic pulmonary hypertension. Chest. 2012; 142:1406–1416.
4. Boerrigter B, Mauritz GJ, Marcus JT, et al. Progressive dilatation of the main pulmonary artery is a characteristic of pulmonary arterial hypertension and is not related to changes in pressure. Chest. 2010; 138:1395–1401.
5. Sahay S, Tonelli AR. Ventricular fibrillation caused by extrinsic compression of the left main coronary artery. Heart. 2013; 99:895–896.
6. Duijnhouwer AL, Navarese EP, Van Dijk AP, Loeys B, Roos-Hesselink JW, De Boer MJ. Aneurysm of the pulmonary artery, a systematic review and critical analysis of current literature. Congenit Heart Dis. 2016; 11:102–109.
7. Sakuma M, Demachi J, Suzuki J, Nawata J, Takahashi T, Shirato K. Proximal pulmonary artery aneurysms in patients with pulmonary artery hypertension: complicated cases. Intern Med. 2007; 46:1789–1793.
8. Choi YJ, Kim U, Lee JS, et al. A case of extrinsic compression of the left main coronary artery secondary to pulmonary artery dilatation. J Korean Med Sci. 2013; 28:1543–1548.
9. Williams TE Jr, Schiller M, Craenen J, Hosier DM, Sirak HD. Pulmonary artery aneurysm. Successful excision and replacement of the main pulmonary artery. J Thorac Cardiovasc Surg. 1971; 62:63–67.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download